Case Report
Subacute infectious endocarditis-associated membranoproliferative glomerular nephritis: A Case Report and Review
Yi De Zhang1, Ya Min Yu2, Hou Yong Dai1, Xiao Lan Chen1, Li Yuan1 and Hui Shi1*
1Nephrology Department of Affiliated Hospital of Nantong University, Nantong, Jiang Su, China
2Nephrology Department of Ningxiang People’s Hospital, Ningxiang, Hu Nan, China
*Address for Correspondence: Dr. Hui Shi, Nephrology Department of Affiliated Hospital of Nantong University, Nantong, Jiang Su, China, Email: [email protected]
Dates: Submitted: 12 June 2017; Approved: 16 August 2017; Published: 17 August 2017
How to cite this article: Zhang YD, Yu YM, Dai HU, Chen XL, Yuan L, et al. Subacute infectious endocarditis-associated membranoproliferative glomerular nephritis: A Case Report and Review. J Cardiol Cardiovasc Med. 2017; 2: 052-055.
DOI: 10.29328/journal.jccm.1001014
Copyright License: © 2017 Zhang YD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Subacute infectious endocarditis; Membranoproliferative glomerulonephritis; Acute renal failure; Antibiotics; Mitral valve replacement
ABSTRACT
We experienced a case of membranoproliferative glomerulonephritis (MPGN) caused by subacute infectious endocarditis (SIE). A 57-year-old male farmer complained of fatigue, lack of appetite and gross haematuria for a month; he had no cough, chest pain, or exertion dyspnea. After admission, lab tests showed mild proteinuria(1.04g/d) and heavy dysmorphic red blood cells(RBC) (543/HP), with serum creatinine(Scr) slightly elevated(1.46mg/dl) and anemia(hemoglobin Hb 85g/L). A renal biopsy revealed MPGN lesion with 16.6% cellular crescents. The echocardiogram test revealed mitra valve prolapse with perforation of the anterior lobe, vegetation, and severe regurgitation. He was diagnosed as SIE induced MPGN. Then he underwent mitral valve replacement after systemic antibiotic treatment without immunosuppressive agents. Follow-up showed that he dramatically regained normal kidney function in total 1 year after the operation. Thus, antibiotic administration and valve replacement may be efficient enough for some of SIE induced MPGN. We did a brief review of the literature on SIE induced MPGN, which was sometimes misdiagnosed due to its silent characteristics; some SIE patients may initially have other organs involved.
INTRODUCTION
Various diseases can lead to proteinuria, haematuria and kidney dysfunction, the etiologies are sometimes latent so that they are difficult to be found and often initially misdiagnosed. SIE can result in kidney infarction, and also is a potential pathogenesis for secondary GN. Baehr G [1], first discovered glomerular lesions of focal and segmental glomerulosclerosis induced by bacterial emboli in a patient with subacute bacterial endocarditis in 1912. However, over half of these SIE patients had no known prior cardiac abnormalities, Asymptomatic patients with SIE may be neglected and misdiagnosed; the delayed treatment sometimes leads to higher mortality rates.
CASE REPORT
3 months ago, a 57-year-old male farmer had an intermittent fever of 38 oC for 2 weeks, without any other symptoms or treatments. 2 months later, he felt fatigue, had a bad appetite, presented slight edema in his lower limbs, as well as gross haematuria; he did not show signs of fever, cough, chest pain or exertion dyspnea. He then went to a local county level hospital, and was admitted. Lab tests revealed leukocytosis and moderate anemia in the blood routine test. The dipstick urinalysis showed a prominent increase of red blood cells, and protein 2+, Scr slightly elevated as 1.46mg/dl, chest X ray examination was normal, kidney ultrasound report showed normal kidney size without any stones. He was diagnosed as primary chronic GN and upper respiratory tract infection, and then was treated with penicillin for 3 days; he suspended all treatment and went back home due to financial difficulties. 1 week later, the symptoms mentioned above exacerbated, so he decided to see doctors at Affiliated Hospital of Nantong University.
At the beginning, he was registered in the outpatient department of hematology because of intermittent fever and anemia. Lab tests showed Scr elevation and then he was transferred to the nephrology department.
After admission to the nephrology department on December 18, 2015, a general physical examination was done. His mental status was normal, height was 168 cm, weight was 58.4 kg, his body temperature was 36.5 oC, pulse rate was 96 beats/min, blood pressure was 125/76mmHg, with Anemic appearance, bilateral pulmonary breath sounds were normal, heart rate was 96 beats/min, a systolic murmur (Levine classification 4/6) at the cardiac apex radiating to the left armpit was heard. Liver and spleen were untouched, slight pitting edema was seen in his lower extremities. Both Osler nodes and Janeway lesions were not observed. He denied past history of hypertension, diabetes, lung or heart diseases, and no drug abuse.
Lab tests showed 2+ proteinuria, 4+ urine occult blood with 543 RBC/HPF, total proteinuria of 1.44g/d, urinary sediment of 4+ dysmorphic RBC. Blood tests revealed a white blood count of 13.5×109/L, Hb of 85g/L, platelet count of 269×109/L, ALT of 33U/L, AST of 45U/L, albumin level of 22.3g/L, globulin level of 43.1g/L(normal range: 25-35), blood urea nitrogen level of 7.6mmol/L(normal range: 3.2-7), Scr level of 1.65mg/dl(normal range: <1.24), uric acid level of 6.6mg/dl, cystatin C of 2mg/L (<1.35), fasting blood glucose level of 5.7mmol/L, total cholesterol level of 3.5mmol/L, hs-CRP level of 84mg/L. The blood electrolyte analysis indicated normal range of K+, Na+ and Cl- levels. Cancer related biomarkers including CEA, PSA, AFP were all in normal range. Anti-hepatitis B and C antibodies, HIV antibody, ANCA, ENA, ANA were all negative, compliment C3 and C4 level were 0.45g/L(normal range:0.7-2.06) and 0.33g/L(normal range:0.11-0.61), respectively. Immunofixation electrophoresis was negative; his blood culture was negative as well.
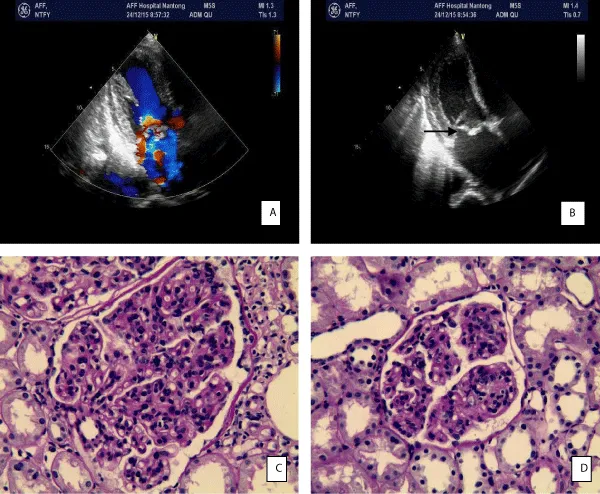
The echocardiogram result revealed mitral valve prolapse with perforation of the anterior lobe, vegetations, and severe regurgitation, his heart function was indicated normal (Figure 1A,B). On the fourth hospital day, the renal biopsy was performed, the immunofluorescent examination showed IgG (2+), IgM (3+), C3 (3+) deposition in the mesangial area and peripheral capillary wall. Light microscope revealed lesions of diffuse proliferative GN pattern, with 16.6% (3/18) of small cellular crescents, infiltration of mononuclear cells were observed. Based on all these data, he was diagnosed as SIE induced MPGN (Figure 1C,D).
Figure 1: mitral valve prolapse with perforation of anterior lobe. Figure B: mitral valve vegetation marked by black arrow heads. Figure C and D: diffuse proliferation with endocapillary hypercellularity.
Antibiotic therapy was carried out soon after he was admitted into the ward; we prescribed intravenous cefoperazone sodium 1.0g/d and teicoplanin 0.2g/d, and kidney function kept stable. Half a month later, he was transferred to the cardiac surgery department for mitral valve replacement on January 3, 2016. After the operation, antibiotics were continuously administered for two weeks. Due to lack of financial support, he returned home again without any further treatment but warfarin.We followed up with this patient for 1 year, his physical situation was very well, and he could undertake various kinds of work. We repeated his blood and urine sample, lab tests showed Scr level of 0.95mg/dl, trace protein and 3-4 RBC/HP by dipstick urinalysis.
DISCUSSION
Cardio renal syndrome (CRS) was well documented over the past decade, diabetes mellitus was one of the leading causes for CRS [2], and our previous research also showed that aldosterone as a critical factor took part in both cardiac and renal injury [3], however, SIE-associated GN is pathogenetically different from CRS. SIE is a common infectious cardiac disease caused by various pathogens such as bacteria, fungus and virus, and patients with heart disease are more susceptible to SIE, especially those with cardiac valvular defect. Other etiological factors include intravenous drug abuse, hepatitis C and diabetes, and the predominant organisms are streptococcal and staphylococci [4]. Blood culture is commonly positive, but occasionally negative [5]. In this case, the patient was considered to be culture-negative SIE. This disease is associated with higher mortality and poor prognosis. SIE-associated GN especially crescentic GN with renal dysfunction is an independent risk factor for mortality [6]. Over the past decades, even if antibiotics are generally used worldwide, the outcomes of SIE did not improve, and the incidence rates of SIE are on the rise worldwide [7]. Over half of SIE patients did not know of their prior cardiac abnormality, these patients may easily be misdiagnosed which may cause treatment delays, taking this case for an example, SIE diagnosis has been delayed several months due to physician’s neglect on heart auscultation.
Up to now, SIE induced renal disease is well established. The mechanisms for SIE-associated kidney involvement include two types; one is kidney infarction due to bacteria emboli occluded in the renal artery, another is secondary GN due to immunologic disarrangement. Mild to moderate normocytic and normochromic anemia was commonly presented, and hypocomplementemia was found in 56% of patients, a small portion of these patients presented ANCA antibody positive [8]. In this case, the patient actually reflected mild normocytic and normochromic anemia and hypocomlementemia. According to literature on renal pathology, the majority of SIE-associated GN types were proliferative glomerularnephritis with endocapillary proliferation and occasional filtrating leukocytes [9]. Previous research showed that crescentic GN was the most common pattern in SIE-associated GN, the second most common pattern was diffuse proliferative GN, and other patterns included mild mesangial hypercellularity without crescent and endocapillary proliferation [8]. However, SIE-associated MPGN was not common according to previous literature; we reported the case of the patient was considered SIE induced MPGN.
Based on the pathogenesis of SIE-associated GN, immuno-disarrangement of complement activation and immunocomplex formation are the triggers, which lead to diffuse proliferation of innate and extrinsic hypercellularity. In this regard, SIE induced GN is a kind of immune complex mediated disease; those patients, especially with crescentic and necrotic lesions, theoretically need immunosuppressant therapy. Meanwhile, the cardiac infection is the trigger for subsequent SIE-associated GN, the controversy between antibiotic and immunosuppressant treatment is obvious, because intensive immunosuppressant agents could exacerbate the infection responsible for SIE [10]. Some researchers suggested combination therapy with antibiotics and corticosteroids simultaneously [11], however, others insisted on isolated antibiotic treatment alone [12]. According to our experiences, therapeutic strategy should be carried out individually.
In this case, we administered antibiotics alone soon after admission instead of combination with immunosuppressive agents because of slight kidney dysfunction and very few small cellular crescents. After mitral valve replacement surgery and further antibiotic treatment, he regained normal renal function after 1 year of follow-up.
In conclusion, SIE-associated GN has increasingly been reported in recent years due to steadily increased infectious rates. Routing physical examination of heart auscultation is mandatory in suspicious SIE patients for avoidance of misdiagnosis. Therapeutic strategy should be conducted individually based on clinical and pathological situations, but antibiotics and valve replacement therapies are imperative for SIE-associated GN.
ACKNOWLEDGEMENT
This research was funded by Nantong regional scientific program of 2016, funding number: MS22016027
REFERENCES
- Baehr G. Glomerular lesions of subacute bacterial endocarditis. J Exp Med. 1912; 15: 330-347. Ref.: https://goo.gl/AMUHUw
- Zhang YD, Dai HY, Xie HL, Zhou QL, Liu ZH. The role of renal damage on cardiac remodeling in patients with diabetic nephropathy. Clin Nephrol. 2013; 80: 249-255. Ref.: https://goo.gl/chLL8F
- Zhang Y, Peng W, Ao X, Dai H, Yuan L, Huang X, et al. TAK-242, a Toll-Like Receptor 4 Antagonist, Protects against Aldosterone-Induced Cardiac and Renal Injury. PLoS ONE. 2015; 10: e0142456. Ref.: https://goo.gl/Tx45dr
- Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 2014; 86: 905-914. Ref.: https://goo.gl/72DX5E
- Naber CK, Erbel R. Infective endocarditis with negative blood cultures. Int J Antimicrob Agents. 2007; 30: 32‑36. Ref.: https://goo.gl/oHVfaC
- Buchholtz K, Larsen CT, Hassager C, Bruun NE. In infectious endocarditis patients mortality is highly related to kidney function at time of diagnosis: a prospective observational cohort study of 231 cases. Eur J Intern Med. 2009; 20: 407‑410. Ref.: https://goo.gl/ztqBVN
- Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S. 1998-2009: a nationwide study. PLoS One. 2013; 8: e60033. Ref.: https://goo.gl/gGjGVt
- Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP. Update on endocarditis-associated glomerulonephritis. Kidney International. 2015; 87: 1241-1249. Ref.: https://goo.gl/UgV7Jg
- Neugarten J, Baldwin DS. Glomerulonephritis in bacterial endocarditis. Am J Med. 1984; 77: 297-304. Ref.: https://goo.gl/7FxBTK
- Kannan S, Mattoo TK. Diffuse crescentic glomerulonephritis in bacterial endocarditis. Pediatr Nephrol. 2001; 16: 423‑428. Ref.: https://goo.gl/KxEBSP
- Krishnamurthy S, Chandrasekaran V, Mahadevan S, Priyamvada PS, Rajesh NG. Severe acute kidney injury in children owing to infective endocarditis-associated immune complex glomerulonephritis: a report of two cases. Paediatr Int Child Health. 2017; 37: 144-147. Ref.: https://goo.gl/mZB7eo
- Manzoor K, Khan S, Ahmed E, Akhter F, Mubarak M, et al. Crescentic glomerulonephritis associated with bacterial endocarditis-Antibiotics alone may be sufficient. A case report. J Pak Med Assoc. 2005; 55: 352-354. Ref.: https://goo.gl/3Zwe4P