Research Article
Non-hemodynamic factors associated to the risk of developing hypertensive cardiopathy
Alexis Álvarez-Aliaga1*, Julio César González-Aguilera2, Liliana del Rosario Maceo-Gómez3, Lic David del Llano Sosa4, Raúl Leyva-Castro5 and Rosa Ojeda-Vázquez6
1Specialist in Internal Medicine. Professor and Researcher. Internal Medicine Department, “Carlos Manuel de Céspedes” General University Hospital, Cuba
2Specialist in Internal Medicine and Intensive and Emergency Medicine. Professor and Researcher. Internal Medicine Department.
3Specialist in General Comprehensive Medicine. Instructor. Internal Medicine Department, “Carlos Manuel de Céspedes” General University Hospital, Cuba
4Professor and Researcher, Specialist in General Comprehensive Medicine, Internal Medicine Department, “Carlos Manuel de Céspedes” General University Hospital, Cuba
5Specialist in Cardiology. Instructor, “Carlos Manuel de Céspedes” General University Hospital, Cuba
6Arterial Hypertension Out-patient Department, Assistant Professor., “Carlos Manuel de Céspedes” General University Hospital, Cuba
*Address for Correspondence: Prof, Alexis Álvarez-Aliaga, MD, PhD, “Carlos Manuel de Céspedes” General University Hospital, Bayamo, Granma, Cuba, Tel: 483034; 425039; Email: [email protected]
Dates: Submitted: 23 August 2017; Approved: 19 September 2017; Published: 20 September 2017
How to cite this article: Álvarez-Aliaga A, González-Aguilera JC, Maceo-Gómez LR, del Llano Sosa JD, Leyva-Castro R, et al. Non-hemodynamic factors associated to the risk of developing hypertensive cardiopathy. J Cardiol Cardiovasc Med. 2017; 2: 068-084.
DOI: 10.29328/journal.jccm.1001017
Copyright License: © 2017 Álvarez-Aliaga A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Arterial hypertension; Hypertensive cardiopathy; Non-hemodynamic cardiovascular risk factors
Abstract
Introduction: Hypertensive cardiopathy is the target organ lesion caused by arterial hypertension (HTN) that exhibits the highest morbidity and mortality rates. Although the importance of hemodynamic overload exerted by HTN on the onset of cardiopathy is well established, several non-hemodynamic factors may contribute significantly to its development.
Objective: To evaluate the influence of different non-hemodynamic risk factors in the development of hypertensive cardiopathy.
Methods: A prospective cohort study was carried out in hypertensive patients assisted at the specialized arterial hypertension physicians’ office of the “Carlos Manuel de Céspedes” Specialty Policlinic attached to the General University Hospital, Bayamo Municipality, Granma Province, Cuba from January 5, 2006 to December 31, 2015. The study included 18-to-55-year-old hypertensive patients with a stage 1 arterial hypertension diagnosis for less than a year1.
Results: The multivariate analysis showed a significant and independent relation among the majority of the factors studied and the risk of developing cardiopathy. The major factor was C-reactive protein (HR: 5.020; IC 95%: 3.383-7,448; p<0.005) followed by microalbuminuria (HR: 2.649; IC 95%: 1.932-3.631; p<0.005). The area under the model ROC curve was 0.887 (p<0,005).
Conclusions: The results showed that it is possible to estimate the risk of developing hypertensive cardiopathy with the application of the regression model to major risk factors.
Introduction
Arterial hypertension (HTN) is defined as a systolic arterial pressure (SAP)≥140 mmHg or a diastolic arterial pressure (DAP)≥90 mmHg, according to evidence obtained from clinical trials which suggest that in patients with these readings of arterial pressure, reductions induced by pharmacological treatment are beneficial [1].
HTN, affects a quarter of the adult population (78 millions of adults in the United States of America and more than 1.000 million people worldwide) [2]. In the year 2016, the prevalence rate of HTN in Cuba was 217.5 per 1000 inhabitants and in Granma Province 168.3 per 1000 inhabitants [3].
This disease is the main cause of death in the world and the major complaint in Out-patient Departments, it is the treatable and most easily recognized risk factor for cerebrovascular accident, myocardial infarction, heart failure, peripheral vascular disease, aortic dissection, auricular fibrillation and terminal nephropathy [2].
Consequently, HTN cannot be considered an isolated condition because, due to its natural evolution, it increases considerably the risk of damage to different organs as well as invalidity and death by cardiovascular causes.
Hypertensive cardiopathy is the target organ damage with the highest morbidity and mortality. It is defined as a group of complex and variable effects which cause chronic arterial pressure elevation in the heart and is characterized by the presence of anatomical and biochemical signs of left ventricular hypertrophy (LVH) or diastolic or systolic ventricular dysfunction, myocardial ischemia and cardiac rhythm alterations [4].
Although the importance of the hemodynamic overload caused by HTN in the development of cardiomyocyte hypertrophy is well known, there are also important pathological processes mediated by hormones, growing factors, cytosines and other inflammatory molecules which, when acting upon the cardiomyocyte and the rest of the cellular and non-cellular components of the myocardium of a hypertensive patient, can explain the development of LVH and myocardial remodeling; a more recent disquisition discloses the discovery of a group of genes involved in the mentioned process [4].
For that reason, it can be stated that HTN control is not always enough to avoid these lesions. Evidence shows that the presence of a number of factors in the hypertensive patient increases the risk of cardiovascular complications in general and hypertensive cardiopathy in particular, and they are independent of HTN control. Among these factors are: age, high intake of sodium in the diet, dyslipidemias, smoking, obesity, sedentariness and the like [1,5-9].
When these factors interact, they can cause hypertensive cardiopathy, but the degree of influence and independence of each has not been clearly defined since diverging results have been found in different studies [4-9].
For all the facts stated above, the objective of the present study is to evaluate the influence of different non-hemodynamic risk factors in the development of hypertensive cardiopathy.
Methodological design
A prospective cohort study was carried out in hypertensive patients assisted at the specialized arterial hypertension physicians’ office of the “Carlos Manuel de Céspedes” Specialty Policlinic attached to the General University Hospital, Bayamo Municipality, Granma Province, Cuba from January 5, 2006 to December 31, 2015. The patients were evaluated in four appointments per year.
Inclusion and exclusion criteria
The study included 18-to-55-year-old hypertensive patients with a stage 1 arterial hypertension diagnosis for less than a year who did not have any clinical or echocardiographic evidence of hypertensive cardiopathy.
For the diagnosis of HTN the measures proposed by the NYHA Seventh Report for the correct determination and classification of blood pressure were taken into account [10].
For the arterial blood pressure determination aneroid and mercury sphygmomanometers, previously calibrated by the Territorial Normalization and Quality Office accredited for this purpose, were used.
Patients with ischemic cardiopathy or interventricular and auroventricular conduction disorders were excluded from the study because the inclusion of subjects with this condition might bias selection and classification, since it might be the result of a non-diagnosed HTN with a longer time of evolution; in addition, it was taken in consideration that the patients did not suffer from other conditions that could cause cardiopathy, such as myocardiopathies (in any clinical form), diabetes mellitus, thyroid diseases, chronic renal failure, and that the patients were not being treated with cytostatic agents or suffered from chronic intestinal inflammatory or collagen diseases.
Likewise, patients who had a diagnosis of hypertensive cardiopathy within the first year of follow-up were excluded from the study.
Patient source
The patients included in the cohort were referred by HTN specialists from different health areas of our province to the hospital HTN Out-patient Department.
Every patient was submitted to detailed interview and physical examination to obtain the necessary data for the study. Later on, clinical evaluations were done every three months based on the analysis of the clinical data gathered; echocardiogram and electrocardiogram were done every six months.
Patients were excluded from the cohort whenever an excluding condition appeared. Each individual evaluation concluded at the end of the study or in the presence of a diagnosis of hypertensive cardiopathy.
During the study all the patients received an initial uniform medical treatment based on the therapeutic protocol approved by the hospital Research Ethics Committee (according to the patient’s age, skin color, risk factors and contraindications. The protocol consisted in the use of an angiotensin II-converting enzyme inhibitor plus a diuretic; calcium channel blocker alone or combined with a diuretic; beta-blocker alone or combined with a diuretic; and it was also kept in mind the latest recommendations about the treatment and management of HTN) [10] to guarantee the control of arterial pressure in the cohort patients and to make as homogeneous as possible the factors which could have any influence in the evolution of the disease and were not considered in the study.
January 5, 2006 was defined as the zero hour or beginning of the cohort. Once the cohort had started it was decided not to include new patients (closed cohort). Every individual evaluation concluded when the patient developed hypertensive cardiopathy or after the ten-year period of the cohort for patients who did not develop the condition.
Sample characteristic
The cohort began with 1363 patients who were followed at the HTN physician’s office for ten years: n=701 patients came from urban areas (51.43%) and n=662 patients came from rural areas (48.57%) of our province. During the study 17 patients died of other causes than hypertensive cardiopathy and 228 dropped out for different reasons (incorrect control of arterial pressure, non-compliance, absences to their evaluation appointments, moving to other places, among others).
Taking into consideration the criteria previously stated, 1118 patients were accepted; 168 (15.02%) of them developed hypertensive cardiopathy: 513 (45.9%) male patients and 513 (54.1%) female patients). The average age was 48 years, 329 (standard deviation: 6.939); while uric acid was the biological marker with greater standard deviation (99.81) (mean: 344.28).
Definition of variables
Dependent variable: onset of hypertensive cardiopathy, every hypertensive patient was diagnosed with this condition provided that in any of the follow-up appointments they began to meet the following criteria:
LVH echocardiographic pattern: Dévereux’s formula was used [11] (LV mass in grams)=0.8 (1.04 [left ventricular diastolic diameter+posterior wall thickness+interventricular septum thickness]3-[left ventricular diastolic diameter])3+0,6. Hypertrophy was considered at a value of ≥125 g/m2 in male and ≥110 g/m2 in female.
Diastolic or systolic dysfunction (by echocardiogram).
Arrhythmia: Persistent atrial fibrillation of long evolution and permanent fibrillation not caused by valve diseases were considered, myocardiopathies, myocarditis, medications and drugs. The diagnosis was based on the patient’s ambulatory clinical history, interview and physical exam. It was later confirmed by a standard twelve-lead electrocardiogram, according to the criteria proposed by the American Cardiology College and the American Heart Association. Electrocardiograms were done by the physician’s office nurse using a portable digital electrocardiograph (CARDIOCID-BB, model A5102).
Echocardiograms were done by two cardiologists with more than 10 years of experience and specialized in echocardiography. An ASAOTE Caris PLUS machine was used following the American Echocardiography Association guidelines.
Independent variables
Included factors associated with the development of hypertensive cardiopathy that were being evaluated and are described below.
Sex was divided into male and female
Smoking was grouped into two categories: (exposed) smokers if they smoked cigarettes, cigars, or pipe daily or almost daily, independent of the number of units smoked and ex-smokers, individuals who had quit the habit less than a year before, and non-smokers, those who had never been in the habit or who had quit it more than a year before the study.
Alcoholism was considered in men who ingested more than an ounce of pure alcohol daily, equivalent to an ounce (20 ml) of ethanol, 8 ounces (240 ml) of wine, 24 ounces (720 ml) of beer, 1½ ounce (45ml) of rum and in the case of women and underweight men those who consumed at least 15 ml per day [12].
Obesity was established by calculating body mass index (BMI greater or equal to 30: weight in kilograms/size in square meters) or a waist circumference greater or equal to 102 cm for men and 88 cm for women, or when both conditions were present.
Sedentariness: Subjects were considered sedentary if they spent daily less than a given amount of time in leisure activities that consume 4 or more metabolic equivalents (physical activity equivalent to or greater than (in energy output) to have a brisk walk (more than 6 km/h) or ride a bicycle at a speed between 16‐19 km/h). The patient’s profession was also taken into account. For the definition of the variable, the patients were asked the questions below, based on the data obtained from studies that related sedentariness and cardiovascular diseases [12,13].
For the definition of variables the patients were asked the questions below taking into consideration data obtained from the analysis of studies that relate sedentariness and cardiovascular diseases [13,14].
1. What kind of job do you do?
Possible answers:
• A job that involves little or no physical activity: usually sits down or stands up all day long or walks very little.
• A job that demands a high energy output from the patient (example: construction workers, farmers, stevedores, etc.).
• What kind of physical activity do you do in your free time?
Possible answers:
• I never exercise.
• Some physical or sports activity (at least 25 minutes for women and 30 for men) several times a month.
• Regular physical activity (at least 25 minutes for women and 30 for men) several times a week.
• Daily physical training.
Patients who answered affirmatively items 1a and 2a were considered sedentary. The rest of them were considered non-sedentary.
Excessive sodium in the diet. Every subject who had an ingestion of salt higher than 5 grams a day (this is equivalent to more than a level teaspoonful, distributed among the dishes prepared for lunch and dinner) was considered exposed. As exposed were also included those patients who consumed bakery products or used table salt (three or more times a week) [12,15]. To obtain more accurate answers, the questions asked about consumed food, according to amount and frequency, the amount of sodium in the foods most frequently consumed, the amount of salt added while cooking and at the table, and the ingestion of foods with high sodium content; to obtain this information the following questions were used:
2. Do you (or anybody else at home) add more than a level teaspoonful of salt per person when cooking, distributed among the dishes prepared for lunch and dinner?
Possible answers:
• Yes
• No
3. Do you add salt to your food after it is cooked or do you use table salt shakers?
Possible answers:
• Yes, every day or almost every day
• Occasionally (less than three times a week)
• Never
4. Do you eat salty foods? Such as preserved foods and sausages (bacon, ham, sardines, olives, canned meat, salami, hot dogs, spiced sausage, and the like); foods that contain salt (crackers, bread, corn flakes, peanut, among others, sauces and canned soups, cheese, butter, mayonnaise, foods preserved in salt); other processed foodstuffs (bottled or carbonated soft drinks, beers, pickles, artificial flavors) or other salty foods (soy sauce, fish sauce, tomato sauce, sauces for local dishes)?
Possible answers:
• Yes, every day or almost every day.
• Occasionally (less than three times a week).
• Never.
Patients who answered affirmatively any of the items 1a, 2a or 3a were considered to have an excessive sodium intake in the diet (exposed).
The biomarkers selected as possible risk factors were cholesterol, uric acid, triglycerides, HDL-cholesterol, glycemia, C-reactive protein (CRP), microalbuminuria and the cholesterol/HDL quotient. Blood samples for laboratory tests were obtained in the fasting state (8 to 12 hours), and they were centrifuged at room temperature at 2000 rpm for 10 minutes. Creatinine, uric acid, cholesterol, HDL-cholesterol, triglycerides, and glycemia were measured with a HITACHI 902® machine during the first 24 hours after extraction and the first two ones were expressed in µmol/l and the rest in mmol/l. Determination of all the studies were done by means of enzymatic methods.
The cutpoints for the values of each quantitative variable were established according to the statistical estimates calculated. Variables were categorized trying to obtain the greatest statistical association and applicability of the results, a method which will be explained later on. As cutpoints for the analysis were considered the following values: serum cholesterol (hypercholesterolemia) over 4.8 mmol/l, triglycerides (hypertriglyceridemia) greater than 1.7 mmol/l, HDL-cholesterol less than 1.5 mmol/l, cholesterol/HDL quotient higher than 4. The cutpoint for glycemia was established when the figures in fasting state reached values greater than 5.4 mmol/l, greater than 80 µmol/l for creatinine, and greater than 375 µmol/l for uric acid.
CRP was determined by the turbidimetric quantitative determination method and values over 4 mg/l were considered a potential risk factor.
Microalbuminuria patients were considered exposed when their values ranged from 0.02 to 0.2 g/l in a 24-hour period [1] and it was quantified by means of the Microalb-Látex technique (measurement of the amount of this substance in morning first-void urine specimen). Negative: suspension that stays homogeneous for the time the technique lasts [3 minutes]. Positive: the presence of agglutination during the analysis indicates that albumin is present in the original sample).
The value of the quantitative variables was based on the average of all the determinations obtained during the cohort. The data were obtained in the appointments during the interviews done by the authors, with the patient’s awareness and consent.
Statistical Analysis
The statistical analysis started with the characterization of the sample and the description of all the variables. The means and standard deviations were determined for the quantitative variables, along with the minimum and maximum values of each distribution. Absolute and relative frequencies (percentages) were obtained for the qualitative variables.
Univariate analysis
Mantel’s chi-square test was used to evaluate the association between the qualitative variables and the risk of developing hypertensive cardiopathy. The magnitude of the associations was estimated by calculating the relative risks (exposed/non exposed) of developing hypertensive cardiopathy in a period between one and ten years. The relative risks were estimated by punctual estimations and by interval of confidence (95%). The hypothesis that the population relative risk was greater than one with a significance level of 0, 05 was proved for each variable.
The same procedure was followed for the univariate analysis of the quantitative variables. The variables were dichotomized looking for optimal cutpoints. In the search of possible cutpoints the most extreme values of the on both sides were not considered, that is, under the 5th and over the 95th percentile. Likewise, due to the likelihood of an increase in type 1 error as a result of the use of several hypothesis tests), the following formula was used to correct it: p=-3.13 minp(1+1.65 Ln(minp)), where minp is the value of minimum probability obtained and p is the corrected value. As optimal cutpoint was taken the value of C, which coincided with the highest result of chi-square obtained (that is to say, the one with the lowest p value) for all the values of the variables that were dichotomized. So it was selected as cutpoint for each variable the value that best sets off the patients who developed hypertensive cardiopathy from those who did not [16].
Multivariate analysis
Cox’s proportional risk model was used with all the independent variables, regression coefficients (β) were estimated, as well as the standard error of each coefficient (SE). The significance for each coefficient was proved (null hypothesis β=0) with Wald’s test and the corresponding chi-square test. The hazard ratios were also determined (hazard ratio, HR) as exp (B) with interval of confidence of 95%. Cox’s proportional risk function permitted to model the risk of developing hypertensive cardiopathy permanently for different covariables. The strategy of staggered selection used was retrograde elimination.
Hazard ratios (HR) estimate how many times the risk of developing hypertensive cardiopathy is greater in exposed subjects compared to non-exposed subjects in each variable, while the rest of the variables are kept under control. The adjustment of Cox’s regression function, which is equivalent to the estimation of its parameters, was dome by the maximum verisimilitude method (SPSS omnibus test). As banned cases were considered all the patients who did not develop hypertensive cardiopathy during the ten-year period that lasted the cohort (both univariate and multivariate analyses were used) and those which met the exclusion criteria were also included in the multivariate analysis as banned data.
The model discriminative capacity was determined by the analysis of the data generated by the ROC curve. Punctual estimations and estimations by interval of confidence (95%) of the area under each curve were done.
Ethical considerations
In the present study all the basic ethical precepts established for the research processes of observational clinical and epidemiological studies were met. The hospital Board of Directors and the Ethical Committee gave their approval for it. The potentially eligible patients were informed about the study and their consent to participate in the study was obtained. Likewise, they were guaranteed that their personal data would not be revealed. The patients also received the corresponding treatment for their disease during the study. None of the patients refused to participate in the study.
Results
The baseline characteristics of the patients are shown in tables 1,2. It can be observed that smoking (457 patients), sedentariness (449 patients) and excessive sodium in the diet (443 patients) were among the most frequent qualitative variables (Table 1).
| Table 1: Sample characterization. Qualitative variables N=1118. | |||
| Variables | Category | Number | (%) |
| Sex | Male Female |
513 605 |
45.9 54.1 |
| Smoking | Yes No |
457 661 |
40.9 59.1 |
| Microalbuminuria | Yes No |
293 825 |
26.2 73.8 |
| Alcoholism | Yes No |
326 792 |
29.2 70.8 |
| Obesity | Yes No |
354 764 |
31.7 68.3 |
| Sedentariness | Yes No |
449 669 |
40.2 59.8 |
| Excessive sodium | Yes No |
443 675 |
39.6 60.4 |
Table 2 represents the average values of the quantitative variables. The Univariate analysis of the qualitative variables is shown in table 3, it can be seen that microalbuminuria was the most important factor to increase the risk of hypertensive cardiopathy (3.577) (IC: 2.720-4.704; p: 0.000) followed by smoking (RR: 2.414; IC: 1.813-3.215; p: 0.000) and excessive sodium in the diet (RR: 2.350; IC: 1.763-3.134; p: 0.000), all significantly.
Table 2: Sample characterization. Quantitative variables N=1118. |
||||
| Variables | Minimum | Maximum | Mean | Standard deviation |
| Age | 19.00 | 55.00 | 48.329 | 6.939 |
| Cholesterol | 2.00 | 9.20 | 4.517 | 0.970 |
| C-reactive protein | 0.10 | 9.30 | 3.773 | 1.756 |
| Uric acid | 109.00 | 765.00 | 331.445 | 92.095 |
| HDL | 0.23 | 2.91 | 1.595 | 0.506 |
| Creatinine | 22.00 | 274.00 | 74.053 | 21.676 |
| Glycemia | 2.60 | 8.40 | 4.555 | 0.897 |
| Triglycerides | 0.13 | 4.77 | 1.591 | 0.646 |
| Cholesterol/HDL | 1.17 | 31.74 | 3.614 | 3.214 |
| Table 3: Univariate analysis of qualitative variables. | ||||||
| Variables | With cardiopathy | Without cardiopathy | Relative risk | Confidence interval (95%) | *p | |
| N= 168 | N= 950 | Inferior | Superior | |||
| No % | No % | |||||
| Microalbuminuria | ||||||
| Yes | 94 32.1 | 199 67.9 | 3.577 | 2.720 | 4.704 | 0.000 |
| No | 74 9.0 | 751 91.0 | ||||
| Smoking | ||||||
| Yes | 103 23.3 | 340 76.7 | 2.414 | 1.813 | 3.215 | 0.000 |
| No | 65 9.6 | 610 90.4 | ||||
| Excessive sodium in the diet | ||||||
| Yes | 104 22.8 | 353 77.2 | 2.350 | 1.763 | 3.134 | 0.000 |
| No | 64 9.7 | 597 90.3 | ||||
| Obesity | ||||||
| Yes | 78 22.0 | 276 78.0 | 1.870 | 1.420 | 2.464 | 0.000 |
| No | 90 11.8 | 674 88.2 | ||||
| Sedentariness | ||||||
| Yes | 83 18.5 | 366 81.5 | 1.455 | 1.102 | 1.921 | 0.008 |
| No | 85 12.7 | 584 87.3 | ||||
| Alcoholism | ||||||
| Yes | 51 15.6 | 275 84.4 | 1.059 | 0.783 | 1.433 | 0.711 |
| No | 117 14.8 | 675 85.2 | ||||
| Sex | ||||||
| Male | 71 13.8 | 442 86.2 | 0.863 | 0.651 | 1.145 | 0.307 |
| Female | 97 15.0 | 508 85.0 | ||||
| *0.000 indicates p<0.001 | ||||||
Table 4 exhibits the univariate analysis of the quantitative variables, its maximum exponent was CRP over 4 and increased the risk of developing hypertensive cardiopathy greater than six (RR: 6.004; IC: 4.142-8.704; p: 0.000) followed by cholesterol/HDL greater than 4 (RR: 3.956; IC: 3.040-5.147; p: 0.000) and cholesterol greater than 4.8 (RR: 2.968; IC: 2.227-3.957; p: 0.000).
| Table 4: Univariate analysis of quantitative variables. | ||||||
| Variables | With cardiopathy |
Without cardiopathy |
Relative risk | Confidence interval (95%) | *p | |
| N= 168 | N= 950 | Inferior | Superior | |||
| No % | No % | |||||
| C-reactive protein greater than 4 mg/l | ||||||
| Yes | 137 28.9 | 337 71.1 | 6.004 | 4.142 | 8.704 | 0.000 |
| No | 31 4.8 | 613 95.2 | ||||
| Cholesterol/HDL greater than 4 | ||||||
| Yes | 81 38.0 | 132 62.0 | 3.956 | 3.040 | 5.147 | 0.000 |
| No | 87 9.6 | 818 90.4 | ||||
| Cholesterol greater than 4.8mmol/l | ||||||
| Yes | 105 26.1 | 297 73.9 | 2.968 | 2.227 | 3.957 | 0.000 |
| No | 63 8.8 | 653 91.2 | ||||
| Glycemia greater than 5mmol/l | ||||||
| Yes | 78 30.5 | 178 69.5 | 2.918 | 2.229 | 3.820 | 0.000 |
| No | 90 10.4 | 772 89.6 | ||||
| Creatinine greater than 99µmol/l | ||||||
| Yes | 97 27.2 | 260 72.8 | 2.912 | 2.203 | 3.850 | 0.000 |
| No | 71 9.3 | 690 90.7 | ||||
| HDL less than 1.5mmol/l | ||||||
| Yes | 96 26.7 | 264 73.3 | 2.807 | 2.125 | 3.709 | 0.000 |
| No | 72 9.5 | 686 90.5 | ||||
| Uric acid greater than 350µmol/l | ||||||
| Yes | 75 27.3 | 200 72.7 | 2.472 | 1.883 | 3.245 | 0.000 |
| No | 93 11.0 | 750 89.0 | ||||
| Triglycerides greater than 1.7mmol/l | ||||||
| Yes | 88 19.3 | 368 80.7 | 1.597 | 1.209 | 2.109 | 0.001 |
| No | 80 12.1 | 582 87.9 | ||||
| *0.000 indicates p<0.001 | ||||||
Table 5 shows the result of the multivariate analysis (Cox’s proporcional regression, retrograde staggered strategy), where can be seen the relation of each variable with the probability of developing hypertensive cardiopathy in years, while the other variables are kept under control. It is observed that the most relevant place was for C-reactive protein (β: 1.613; HR: 5.020; IC del 95%: 3.383-7.448) followed in order of significance by microalbuminuria (β: 0.974; HR: 2.649; IC del 95%: 1.932-3.631) and total cholesterol (β: 0.826; HR: 2.284; IC del 95%: 1.637-3.188).
| Table 5: Multivariate analysis. Cox’s proportional regression. | ||||||
| Variables | *β | †SE | p | HR | CI (95%) | |
| Inferior | Superior | |||||
| C-reactive protein>4mg/l | 1.613 | 0.201 | 0.000 | 5.020 | 3.383 | 7.448 |
| Microalbuminuria | 0.974 | 0.161 | 0.000 | 2.649 | 1.932 | 3.631 |
| Total cholesterol>4.8mmol/l | 0.826 | 0.170 | 0.000 | 2.284 | 1.637 | 3.188 |
| Glycemia>5mmol/l | 0.798 | 0.161 | 0.000 | 2.221 | 1.620 | 3.046 |
| Cholesterol/HDL>4 | 0.741 | 0.170 | 0.000 | 2.099 | 1.503 | 2.931 |
| Creatinine>99µmol/l | 0.575 | 0.174 | 0.001 | 1.777 | 1.264 | 2.499 |
| Smoking | 0.542 | 0.169 | 0.001 | 1.720 | 1.235 | 2.394 |
| Excessive sodium in the diet | 0.403 | 0.170 | 0.017 | 1.497 | 1.073 | 2.088 |
| HR: Hazard ratio. CI (95%): Confidence Interval (95%) *Model’s estimated coefficients †Standard error of coefficients |
||||||
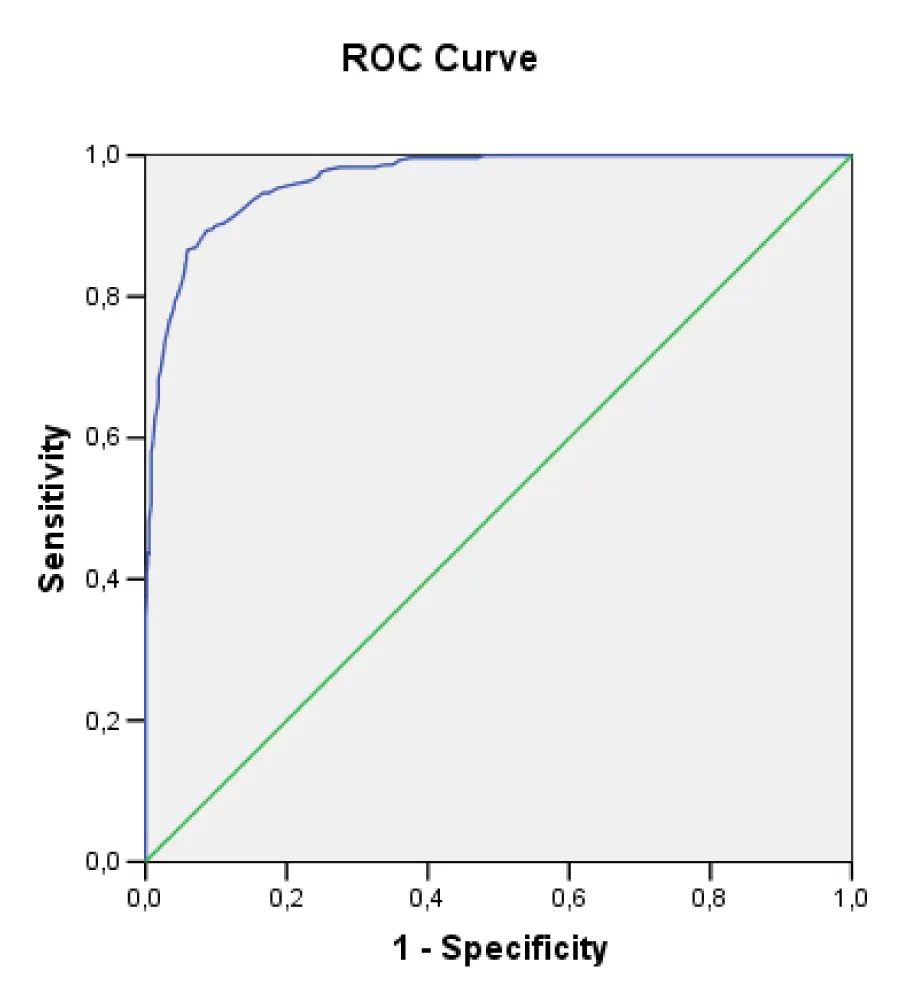
To estimate the model discriminative capacity, the ROC curve (Figure 1) was determined and an area under the curve of 0.887 (IC of 95%: 0.863-0.912; p: 0.000) was obtained. These results show that the capacity of the model to discriminate the probability of developing hypertensive cardiopathy better than chance is significantly higher.
| Area under the curve. | ||||
| Area | Standard Error |
p | Confidence Interval (95%) | |
| Inferior | Superior | |||
| 0.887 | 0.013 | 0.000 | 0.863 | 0.912 |
Discussion
Hypertensive cardiopathy comprises a broad spectrum of complications such as LVH, left ventricle systolic and diastolic dysfunction which may bring about a cardiac insufficiency syndrome, sudden death, myocardial ischemia and arrhythmias, mainly due to the hemodynamic influence exerted by arterial blood pressure on the myocardium [10,17,18]. However, HTN control is not always enough to reduce the risk of cardiopathy, which means that there are several factors involved in its genesis [4,18].
Hypertensive cardiopathy results from several simultaneous interwoven factors, so an intensive integrated approach is required to identify the risk, in order to reduce the long-term overwhelming clinical burden. In the context of the present study a group of non-hemodynamic factors that increase the risk to develop this condition were identified.
Microalbuminuria is considered a cardiovascular risk marker. In addition, a high prevalence of concentric LVH and subclinical deterioration of the left ventricle has been found in subjects with microalbuminuria [19,21]. These findings are consistent with the present study.
The close relationship between microalbuminuria and physiopathological disorders such as: resistance to insulin, endothelial dysfunction, dyslipidemias, sensitivity to salt and angiotensine II increase; cause in endothelial cells, an inflammatory process that activates proinflammatory and inflammatory mediators and growth factors, which bring about dysfunction of cell membranes, not only in the kidneys but also in the heart [22,23], an aspect which can explain our findings.
In conclusion, microalbuminuria not only distinguishes between the benign increase of the glomerular blood flow and glomerular capillary hypertension, but also foretells the later development of endothelial dysfunction, arterial remodeling, LVH, heart failure, generalized atherosclerosis and premature death [24].
Smoking, a well-known cardiovascular risk factor, induces endothelial dysfunction and vasoconstriction which may be related to the result of the independent association between smoking and the lack of control of arterial blood pressure, but in controlled patients it also plays a role in the development of hypertensive cardiopathy since it provokes an increase in platelet aggregation, increases the production of oxygen and cytosine free radicals, which contributes to the formation of macrophages and lipid core; nicotine activates the sympathetic nervous system, one of the physiopathological mechanisms of arterial hypertension and interferes in the action of several antihypertensive drugs [4,24-26], facts which explain the findings of this study.
Direct indications of association between sodium intake and cardiovascular diseases have been scanty and, sometimes, paradoxical, mainly due to methodological difficulties [28]. However, in a recent meta-analysis of prospective observational studies [29] a higher sodium intake was associated to a greater risk of cardiovascular diseases. Another study has demonstrated that a reduced sodium intake diminishes the risk of developing cardiac failure [30].
The most harmful is the salt added to cooked foods (table salt in restaurants or salty foods like sausages and pastas) [31].
A reduced sodium intake can have beneficial effects independent of the effects that it can cause on arterial blood pressure. It reduces the risk of developing subclinical cardiovascular diseases (LVH, ventricular fibrosis and diastolic dysfunction) [32].
Hypertensive and obese patients have a twofold risk of developing LVH4. Cuspidi [33] showed that obesity nearly doubled the risk of biventricular hypertrophy; while Ärnlöv [34] found it as the main risk factor among the components of the metabolic syndrome for cardiovascular diseases.
Obesity is a risk factor for atherosclerosis due to several mechanisms; among them outstand resistance to insulin, its common association with the metabolic syndrome, produces dysglycosis, dyslipidemias, endothelial dysfunction and inflammation caused by a cytosine unbalance (the harmful ones are secreted in excess [interleukins 6 and 18, alpha tumoral necrosis factor and leptin among the most important ones] and a scanty secretion of the protective cytosines [adiponectine]) [35]. On the other hand, genetic changes and the over expression of the activated alpha receptor of the proliferated peroxisome, promote lipid accumulation in the heart and the development of cardiomyopathy [36]. These data support the findings of the present study.
Another factor associated to hypertensive cardiopathy is sedentariness. The data and extensive clinical experience have revealed the importance of physical exercise to influence positively the function of the left ventricle and the arthroscleroses process, as well as to improve the prognosis after a coronary event [37].
Moderate physical exercise improves cardiac function and attenuates the development of HTN and its complications, as it decreases oxidative stress and inflammation, through changes in the effects of the sympathetic nervous system [38]. These evidences are coherent explanations to our results.
Differently to the findings of the present study, others authors found an association between alcoholism and the risk of cardiovascular complications in hypertensive patients. For instance, a transversal analysis of moderate-to-severe Chinese adult drinkers showed greater cardiovascular complications in comparison to teetotalers [39]; Núñez-Córdoba [40], found greater risk of HTN related to the kind of beverage (hazard ratio=1.18) and the amount ingested (hazard ratio=1.45) while the red wine consumption showed to be a protective factor for cardiovascular events.
However, a slight consumption of alcohol is associated to a decrease in the incidence and mortality of coronary diseases, due to the elevation of HDL cholesterol and the favorable effect on homeostasis and its antithrombotic effect, contrary to high consumption which may bring serious cardiovascular complications such HTN and alcoholic cardiopathy, which combined can worsen the prognosis [41]. These aspects suggest a probable protective effect of a slight ingestion.
Gender was not a major risk for the development of hypertensive cardiopathy. Although arterial hypertension appears earlier in men and is associated to other cardiovascular risk factors, it has been observed that after menopause, the risk is the same for both sexes and it can be even greater in women9. In the authors’ view, at present, least in our country, women share many risk factors and toxic habits which were more frequent in men in the past.
C-reactive protein is a protein of the acute phase of inflammation, but it is also considered a biomarker of risk for cardiovascular diseases (HTN and heart failure). Its elevation is associated with left ventricular dysfunction and an increase of both the morbidity and mortality in heart failure patients independent of its etiology [42-45].
Laboratory findings suggest that it is not only a biomarker but it also regulates the AT1 receptors of angiotensin and stimulate the production of proinflammatory cytosines and attenuates the production of nitric oxide [46,47]. Inflammation is an early process in hypertensive cardiopathy, which can explain the importance of C-reactive protein as a predictive factor in this study. It has been demonstrated that high values of C-reactive protein accelerate dilatation and LVH induced by pressure overload, as well as an increase of apoptosis, perivascular fibrosis and endothelial dysfunction; furthermore, it increases the production of 1-β interleukin, which reduces the bioavailable nitric oxide (by inhibition of its enzyme) with hypoxia and apoptosis of the cardiomyocytes and favors fibrosis and the development of hypertensive cardiopathy [42,48].
Nagai [42], stated that elevated values of C-reactive protein worsen the cardiac remodeling caused by pressure overload. It was also associated with an increase of proinflammatory cytosines, deficiency in the production of nitric oxide and greater activity of the rennin-angiotensin system. These factors had a synergic presentation and became a vicious circle, where C-reactive protein could be the key factor in the physiopathological phenomenon.
Finally, in a study that examined the relation between the increase of cardiac remodeling and the elevated values of C-reactive protein, it was demonstrated that the anti-inflammatory treatment with eicosapentanoic acid prevented the development of cardiac failure, particularly that induced by pressure overload [49].
These evidences would explain the role of C-reactive protein in the genesis of hypertensive cardiopathy.
Lipid metabolic disorders, in general, have been associated for more than 70 years to the risk of cardiovascular diseases and their complications, particularly with HTN and ischemic cardiopathy; however, the implication in the risk of each component (total cholesterol, triglycerides, HDL and LDL) is different and can change with age [50].
Both cholesterol/HDL coefficient and total cholesterol are related to the worst prognosis in hypertensive patients. Piskorz [51], showed that elevated values of total cholesterol increased the risk of LVH significantly. Other studies have stated the direct association between elevated values of total cholesterol and the probability of cardiovascular diseases and LVH [33,52,53]. Although these results coincide with the eons obtained in the present series, it should be pointed out that in the latter was found a greater association with the risk of developing hypertensive cardiopathy, probably as a result of the cutpoints used that allowed a greater sensitivity and specificity to this variable.
Lipid metabolic disorder is one of the most important risk factors for HTN and its complications; its value resides in the effects on the acceleration of arthroscleroses of both conditions that are boosted exponentially when they are concomitant in the same subject [52]. Regarding glucose metabolism, Cuspidi [53], showed that values over 5.2 mmol/L in fasting non-diabetic but hypertensive people was an independent risk factor (OR=1.28) for hypertensive cardiopathy. In diabetic patients, Felício [54], found association between nocturnal hyperglycemia and LVH; in addition, he stated that glucose values of 6.05 mmol/L or higher in fasting, increased the risk of cardiovascular diseases 1.33 times compared to figures of 4.16 mmol/L in the same condition.
In the present series the tendency observed was similar, with cutpoints for glycemia similar to the authors quoted.
Likewise, chronic hyperglycemia leads to the accumulation of the final products of advanced non-enzymatic glycation, where reductive sugars modify collagen and other proteins, causing a greater rigidity of the myocardium; besides, resistance to insulin, common in these patients, predisposes for lipotoxicity which can cause dysfunction of the myocytes due to an increase of oxidative stress, mitochondrial disengagement and apoptosis [54].
Hyperglycemia associated to HTN, can cause cardiac hypertrophy and interstitial fibrosis, arthroscleroses and coronary endothelial dysfunction which predisposes for ischemia if stenosis of these arteries is absent. There is also a greater activation of the sympathetic nervous system, and alterations of the systolic and diastolic functions occur in absence of valvular or coronary diseases [56,57]. Therefore, a glycemia level even below the threshold for the diagnosis of diabetes mellitus, is undoubtedly an important risk factor for the development of hypertensive cardiopathy. Similarly to the present study, other authors found creatinine levels below the threshold for chronic renal disease in patients with HTN and damage to target organs [58,59].
The kidney plays an important role in the onset and development of HTN, where a group of complex and interwoven factors which include the rennin- angiotensin-aldosterone system, several inflammatory mediators and reactive species of oxygen, provoke histological and functional changes characteristic of the hypertensive renal lesion. Finally, renal and cardiac tubulointerstitial fibrosis takes place and, as a consequence, dysfunction of both organs [60,61]. Concretely, it would be another indicator of universal endothelial functional and structural alteration and of prediction of hypertensive cardiopathy.
Current HTN guidelines [1,63], recommend an adequate lipid control, since a reduction in the incidence of cardiovascular diseases has been demonstrated in patients with low plasma levels of this substance and the benefit of the treatment with astatines; however, few studies evaluate the risk of hypertensive cardiopathy in patients with low HDL.
Cuspidi [33], Barter [52] and Yeboah [63], for instance, found greater risk of LVH in subjects with low HDL, which is consistent with the present study.
Although many physiopathological elements have already been explained when dealing with the importance of total cholesterol, as well as the cholesterol/HDL coefficient in the genesis of the damage to target organs by HTN, it should be highlighted that high levels of HDL reduce atherosclerosis and cardiovascular complications, and for that reason they are considered therapeutic targets, independently of the reduction of LDL levels. It has been observed that in patients treated with astatines cardiovascular risk has not been reduced when HDL remains low [64]. Elevated levels of uric acid was a risk factor for hypertensive cardiopathy, and some studies [33,55], coincide with our findings.
Experiments in animals have permitted to identify some of the mechanisms by which uric acid can induce cardiovascular diseases, among which are a stimulus to the rennin-angiotensin-aldosterone system and a decrease of the endothelial production of nitric oxide. Extended studies in humans suggest that this substance could be related to the onset of HTN. This is, undoubtedly, a manifestation of intense endothelial dysfunction, metabolic syndrome, oxidative stress and increase of rennin activity in hypertensive individuals [65,66]. In fact, uric acid is not an inert molecule, but a molecule capable of inducing a proinflammatory state and activating the rennin-angiotensin-aldosterone system at a vascular level [66].
As in the present series, Cuspidi [55] and Yeboah [63], did not find any non-independent association between high levels of triglycerides and the risk of LVH.
The results can be explained by the association of these lipids with atherosclerosis, endothelial dysfunction and cardiac remodeling. They can increase or reduce the supply of sterol, which causes an atherogenic inflammatory response; besides, high levels of plasma triglyceride are associated with an HDL concomitant decrease and with the insulin resistance la syndrome; both are markers of metabolic disorders that contribute to a bad prognosis in hypertensive patients and predict the risk for LVH [67,68].
The present study concludes by demonstrating the relevant role played by C-reactive protein, microalbuminuria, total cholesterol and glycemia levels as the main non-hemodynamic risk factors to develop hypertensive cardiopathy. During the analysis of each factor, their influence and importance in the genesis of hypertensive cardiopathy were clearly explained.
The results obtained with Cox’s proportional regression allowed the analysis of the area under the ROC curve, which demonstrate that the model, based on the most important factors discriminates better than chance the risk to develop hypertensive cardiopathy.
As limitations of the present study it should be pointed out that it was not possible to study new predictors of cardiovascular risk such as hypersensitive C-reactive protein, endostatin, homocysteine, A and B apoliproteins, among others. Likewise, it was not possible to quantify urine sodium to evaluate sodium intake objectively.
References
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, et al. Guía de práctica clínica de la ESH/ESC 2013 para el manejo de la hipertensión arterial. RevEspCardiol. 2013; 23: 3-16. Ref: https://goo.gl/pdLH5T
- Victor RG. Arterial hypertension. En: Lee Goldman L, Schafer AI, editors. Goldman-Cecil Medicine. 25th ed.Philadelphia, PA: ElsevierSaunders. 2016; 67: 381-397.
- República de Cuba. Ministerio de Salud Pública. Anuario Estadístico de Salud. 2016.
- Díez J, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010; 55: 1-8. Ref: https://goo.gl/NyEqK8
- Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GDO, et al. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007; 115: 2119-2127. Ref: https://goo.gl/hzbp9m
- Sharman JE. New insights into cardiovascular risk from the exercise central waveform. Artery Res. 2009; 2: 132-137. Ref: https://goo.gl/BpS9FK
- Tomiyama H, Matsumoto C, Yamada J, Yoshida M, Odaira M, et al. Predictors of progression from prehypertension to hypertension in Japanese men. Am J Hypertens. 2009; 22: 630-636. Ref: https://goo.gl/QXDu2D
- Álvarez Aliaga A, González Aguilera JC, Maceo Gómez LR. Factores asociados al desarrollo de la cardiopatía hipertensiva: un estudio de cohorte, en Bayamo, Cuba. Medwave2016; 16: 6492. Ref: https://goo.gl/KjbCaF
- Álvarez Aliaga A, González Aguilera JC. Algunos factores de riesgo de la cardiopatía hipertensiva. Rev Cubana de Med. 2009; 48: 139-151. Ref: https://goo.gl/LZnLPD
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289: 2560-2572. Ref: https://goo.gl/21maJJ
- Dévereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977; 55: 613-618. Ref: https://goo.gl/N3sdsr
- Pérez Caballero MD, Dueñas Herrera A, Alfonso Guerra JP, Vázquez Vigoa A, Navarro Despaigne D, et al. Hipertensión arterial. Guía para la prevención, diagnóstico y tratamiento. Comisión Nacional Técnica Asesora del Programa de Hipertensión Arterial. La Habana: Editorial Ciencias Médicas. 2008.
- Whelton S, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002; 136: 493-503. Ref: https://goo.gl/8jtdSP
- Cabrera de León A, Rodríguez-Pérez C, Rodríguez-Benjumeda M, Anía-Lafuente B, Brito-Díaz B, et al. Sedentarismo: tiempo de ocio activo frente a porcentaje del gasto energético. RevEspCardiol. 2007; 60: 244-50. Ref: https://goo.gl/EpUDwi
- Zhao W, Hasegawa K, Chen J. Part A. Recent advances in dietary assessment tools. The use of food-frequency questionnaires for various purposes in China. Public Health Nutrition. 2002; 5: 829-833. Ref: https://goo.gl/M3zAAt
- Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000; 19: 113-132. Ref: https://goo.gl/A7MNWm
- Graf K, Schaefer-Graf UM. Is Smad3 the key to inflammation and fibrosis in hypertensive heart disease? Hypertension. 2010; 55: 1088-1089. Ref: https://goo.gl/KnonvS
- Drazner MH. The progression of hypertensive heart disease. Circulation. 2011; 123: 327- 334. Ref: https://goo.gl/AVmvg5
- Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, et al. Which markers of subclinical organ damage to measure in individuals with high normal blood pressure? J Hypertens. 2009; 27: 1165-1171. Ref: https://goo.gl/jXron1
- Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, et al. The Relationship between proteinuria and coronary risk: A systematic review and meta-analysis. PLoSMed. 2008; 5: 1486-1495. Ref: https://goo.gl/J8fLJC
- Maione A, Annemans L, Strippoli G. Proteinuria and clinical outcomes in hypertensive patients. Am J Hypertens. 2009; 22: 1137-1147. Ref: https://goo.gl/LxKXLg
- Zhang Z, Dzau VJ. Angiotensin II type 1 receptor–associated protein is an endogenous inhibitor of angiotensin II type 1 receptor action in cardiac hypertrophy. Role in check and balance. Hypertens. 2010; 55:1086-1087. Ref: https://goo.gl/RMHN9T
- Tocci G, Paneni F, Ponziani B, Volpe M. Use of predictive markers to improve cardiovascular protection. FutureCardiol. 2007; 3: 447-456. Ref: https://goo.gl/Axptnn
- Martínez-Castelao A, Górriz JL, Bover J, Segura-de la Morena J, Cebollada J, et al. Documento de consenso para la detección y manejo de la enfermedad renal crónica. Hipertens Riesgo Vasc. 2014; 46: 501-519. Ref: https://goo.gl/ki1f1w
- Halperin RO, Michael Gaziano JM, Howard D, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertens. 2008; 21: 148-152. Ref: https://goo.gl/6DK9ug
- Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, et al. Effects of smoking and smoking cessation on endothelial function 1-year outcomes from a randomized clinical trial. J Am CollCardiol. 2010; 55: 1988-1995. Ref: https://goo.gl/jW5LjH
- Schmidt AC, Flick B, Jahn E, Bramlage P. Effects of the vasodilating beta-blocker nebivolol on smoking-induced endothelial dysfunction in young healthy volunteers. Vasc Health Risk Manag. 2008; 4: 909-915. Ref: https://goo.gl/Mg88bR
- Appel LJ. The case for population-wide salt reduction gets stronger. BMJ. 2009; 339:b4980. Ref: https://goo.gl/rcBr9T
- Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009; 339: 4567. Ref: https://goo.gl/6863jC
- He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, et al. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey epidemiologic follow-up study. Arch Intern Med. 2002; 162: 1619-1624. Ref: https://goo.gl/C3UzFE
- Haddy FJ. Role of dietary salt in hypertension. LifeSci. 2006; 79: 1585-1592. Ref: https://goo.gl/WFoaYs
- Frohlich ED. The salt conundrum: a hypothesis. Hypertension. 2007; 50: 161-166. Ref: https://goo.gl/p6RdEQ
- Cuspidi C, Valerio C, Sala C, Negri F, Esposito A, et al. Metabolic syndrome and biventricular hypertrophy in essential hypertension. J Hum Hypertens. 2009; 23: 168-175. Ref: https://goo.gl/xpZyxA
- Ärnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010; 121: 230-236. Ref: https://goo.gl/gpZ9vq
- Alegría Ezquerra E, Castellano Vázquez JM, Alegría Barrero A. Obesidad, síndrome metabólico y diabetes: implicaciones cardiovasculares y actuación terapéutica. RevEspCardiol. 2008; 61: 752-764. Ref: https://goo.gl/QoQnss
- Son NH, Yu S, Tuinei J, Arai K, Hamai H, et al. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010; 120: 3443-3454. Ref: https://goo.gl/fEM9c6
- Hernández del Rey R. ¿Es importante el ejercicio físico en el control del hipertenso? En: Coca A, de la Sierra A, editores. Decisiones clínicas y terapéuticas en el paciente hipertenso. 3a ed. Barcelona: JIMS SL. 2002; 313-320.
- Agarwal D, Haque M, Sriramula S, Mariappan N, Pariaut R, et al. Role of proinflammatory cytokines and redox homeostasis in exercise induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension. 2009; 54: 1393-1400. Ref: https://goo.gl/CRx6g5
- Wildman RP, Gu D, Muntner P, Huang G, Chen J, et al. Alcohol intake and hypertension subtypes in Chinese men. J Hypertens. 2005; 23: 737-743. Ref: https://goo.gl/Q6MHyS
- Núñez-Córdoba JM, Martínez-González MA, Bes-Rastrollo M, Toledo E, Beunza JJ, et al. Consumo de alcohol e incidencia de hipertensión en una cohorte mediterránea: el estudio SUN. RevEspCardiol. 2009; 62: 633-641. Ref: https://goo.gl/6bNdSb
- Fernández-Solá J, Fatjó F, Sacanella E, Estruch R, Bosch X, et al. Evidence of apoptosis in alcoholic cardiomyopathy. Hum Pathol. 2006; 37: 1100-1105. Ref: https://goo.gl/pN7Bxh
- Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, et al. C-reactive protein overexpression exacerbates pressure overload–induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011; 57: 208-215. Ref: https://goo.gl/vbi3iP
- Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005; 112: 1428-1434. Ref: https://goo.gl/GQr6pD
- Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, et al. C-reactive protein and risk of heart failure. The Rotterdam study. Am Heart J. 2006; 152: 514-520. Ref: https://goo.gl/yB1JDM
- Araujo JP, Lourenco P, Azevedo A, Frioes F, Rocha-Goncalves F, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Cardiol Fail. 2009; 15: 256-266. Ref: https://goo.gl/JqbyBW
- Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003; 107: 1783-1790. Ref: https://goo.gl/58YVLT
- Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002; 106: 913-919. Ref: https://goo.gl/e8TDqs
- Zhang R, Zhang YY, Huang XR, Wu Y, Chung AC, et al. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease. Hypertension. 2010; 55: 953-960. Ref: https://goo.gl/dH6z2b
- Duda MK, O’Shea KM, Tintinu A, Xu W, Khairallah RJ, et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009; 81: 319-327. Ref: https://goo.gl/4DbFwi
- Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, et al. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. CircCardiovascImaging. 2009; 2: 191-198. Ref: https://goo.gl/Wmxaxf
- Piskorz D, Quaglino M, Pigozzi F, Vitelleschi M. Importancia de las variables no hemodinámicas en el desarrollo de hipertrofia ventricular izquierda en hipertensión. RevFedArgCardiol. 2010; 39: 288-293. Ref: https://goo.gl/eEtSJo
- Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007; 27: 1301-1310. Ref: https://goo.gl/21LQVF
- Ashen MD, Blumenthal RS. Clinical practice. Low HDL cholesterol levels. N Engl J Med. 2005; 353: 1252-1260.
- O’Donnell CH, Elosua R. Factores de riesgo cardiovascular. Perspectivas derivadas del FraminghamHeartStudy. RevEspCardiol. 2008; 61: 299-310. Ref: https://goo.gl/9cVUxP
- Cuspidi C, Giudici V, Negri F, Meani S, Sala C, et al. Improving cardiovascular risk stratification in essential hypertensive patients by indexing left ventricular mass to height2.7. J Hypertens. 2009; 27: 2465-2471. Ref: https://goo.gl/dAW2xq
- Felício JS, T Pacheco JT, Ferreira SR, Plavnik F, Moisés VA, et al. Hyperglycemia and nocturnal systolic blood pressure are associated with left ventricular hypertrophy and diastolic dysfunction in hypertensive diabetic patients. CardiovasDiabetol. 2006; 5: 19. Ref: https://goo.gl/KbSxEy
- Franjic B, Marwick TH. The diabetic, hypertensive heart: epidemiology and mechanisms of a very high-risk situation. J HumHypertens. 2009; 23: 709-717. Ref: https://goo.gl/QhoJfF
- Oliveras A, Armario P, Hernández-del Rey R, Arroyo JA, Poch E, et al. Urinary albumin excretion is associated with true resistant hypertension. J HumHypertens [Internet]. 2010; 24: 27-33. Ref: https://goo.gl/GQCpFf
- Xu JZ, Zhang Y, Wu SN, Niu WQ, Zhu DL, et al. Impaired endothelial function in hypertensive patients with target organ damage. J HumHypertens. 2009; 23: 751-757. Ref: https://goo.gl/q51Vu6
- De Leeuw PW, Ruilope LM, Palmer CR, Brown MJ, Castaigne A, et al. Clinical significance of renal function in hypertensive patients at high risk: results from the INSIGHT trial. Arch Intern Med. 2004; 164: 2459-2464. Rerf: https://goo.gl/5B4nux
- Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, et al. The Role of Inflammatory Markers in the Cardioprotective Effect of l-Carnitine in l-NAME-Induced Hypertension. Am J Hypertens. 2008; 21: 1231-1237. Ref: https://goo.gl/Q6pT3Z
- James PA, Oparil S, Carter BL, Cushman WC, Dennison- Himmelfarb C, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311: 507-520. Ref: https://goo.gl/pGxtdB
- Yeboah J, Crouse JR, Bluemke DA, Lima JAC, Polak JF, et al. Endothelial dysfunction is associated with left ventricular mass (assessed using MRI) in an adult population (MESA). J Hum Hypertens. 2011; 25: 25-31. Ref: https://goo.gl/HBjT9z
- Badimón JJ, Ibáñez B. Incremento de las HDL como arma terapéutica en la aterotrombosis. RevEspCardiol. 2010; 63: 323-333. Ref: https://goo.gl/2sbhco
- Feig DI, Kang DH, Johnson RJ.Uric Acid and Cardiovascular Risk. N Engl J Med. 2008; 359: 1811-1821. Ref: https://goo.gl/8ZNnZL
- Vlachopoulos C, Xaplanteris P, Vyssoulis G, Bratsas A, Baou K, et al. Association of serum uric acid level with aortic stiffness and arterial wave reflections in newly diagnosed, never-treated hypertension. Am J Hypertens. 2011; 24: 33-39. Ref: https://goo.gl/ZoZpJk
- Brunzell JD. Hypertriglyceridemia. N Engl J Med. 2007; 357: 1009-1017. Ref: https://goo.gl/kPvZw5
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, et al. Triglycerides and cardiovascular disease a scientific statement from the American Heart Association. Circulation. 2011; 123: 2292-2333. Ref: https://goo.gl/sDr9tG