More Information
Submitted: 13 August 2019 | Approved: 21 August 2019 | Published: 22 August 2019
How to cite this article: Miao J, Estis J, Su YR, Todd JA, Lenihan DJ. Role of novel cardiac biomarkers for the diagnosis, risk stratification, and prognostication among patients with heart failure. J Cardiol Cardiovasc Med. 2019; 4: 103-109.
DOI: 10.29328/journal.jccm.1001049
Copyright License: © 2019 Miao J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Role of novel cardiac biomarkers for the diagnosis, risk stratification, and prognostication among patients with heart failure
Jennifer Miao1, Joel Estis2, Yan Ru Su3, John A Todd2 and Daniel J Lenihan4*
1Vanderbilt University Medical Center, Department of Internal Medicine, Nashville, TN, USA
2Singulex, Alameda, CA, USA
3Vanderbilt University Medical Center, Division of Cardiovascular Medicine, Nashville, TN, USA
4Washington University in St. Louis, Cardiovascular Division, St. Louis, MO, USA
*Address for Correspondence: Daniel J Lenihan, MD, FACC, Professor, Cardiovascular Division, Washington University in St Louis, 660 South Euclid Avenue, campus box 8086, St. Louis, MO 63110-1093, USA, Tel: 314 273 2425; Email: [email protected]
Background: Current guidelines for diagnosis and management of heart failure (HF) rely on clinical findings and natriuretic peptide values, but evidence suggests that recently identified cardiac biomarkers may aid in early detection of HF and improve risk stratification. The aim of this study was to assess the diagnostic and prognostic utility of multiple biomarkers in patients with HF and left ventricular systolic dysfunction (LVSD).
Methods: High-sensitivity cardiac troponin I (cTnI), N-terminal pro b-type natriuretic peptide (NT-proBNP), interleukin-6 (IL-6), endothelin-1 (ET-1), pro-matrix metalloproteinase-9 (pMMP-9), and tumor necrosis factor-alpha (TNF-α) were measured using single-molecule counting technology in 200 patients with varying stages of HF. Plasma detection with cross-sectional associations of biomarkers across all HF stages, and advanced-therapy and transplant-free survival were assessed using multivariate analysis and Cox regression analyses, respectively.
Results: NTproBNP, pMMP-9, IL-6 were elevated in early, asymptomatic stages of HF, and increased with HF severity. Higher circulating levels of combined IL-6, NTproBNP, and cTnI predicted significantly worse survival at 1500-day follow-up. Cox regression analysis adjusted for ACC/AHA HF stages demonstrated that a higher concentration of IL-6 and cTnI conferred greater risks in terms of time to death, implantation of left ventricular assist device (LVAD), or heart transplantation.
Conclusion: Biomarkers of inflammation, LV remodeling, and myocardial injury were elevated in HF and increased with HF severity. Patients had a significantly higher risk of serious cardiac events if multiple biomarkers were elevated. These findings support measuring NTproBNP, cTnI and IL-6 among patients with HF and LVSD for diagnostic and prognostic purposes.
Heart failure (HF) is a leading cause of hospitalizations among adults and the elderly in the US, with estimated medical costs expected to increase from $20.9 billion in 2012 to $53.1 billion by 2030 [1]. The mean per-patient cost of an HF-related hospitalization in the US was $10,775 in 2011, and the prevalence of HF is estimated to be 5.7 million with an incidence of 870,000 new cases diagnosed every year. Approximately fifty percent of patients with HF are expected to die within five years of diagnosis [2]. HF thus confers not only a substantial burden on patients, but the hospitalization rates are at epidemic proportions and strategies are needed that can direct prevention, early detection and successful, tailored therapy. The current ACC/AHA guidelines for the management of HF define the condition as a “complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood” and that practically there is a lack of a “single diagnostic test for HF because it is largely a clinical diagnosis based on a careful history and physical examination” [3]. Furthermore, studies have identified the limited reliability and decreased specificity of physical exam findings (jugular venous distention, rales, edema) for the diagnosis of HF [4,5]. Natriuretic peptides (i.e. NT-proBNP) have been identified as biomarkers that assess the presence and severity of HF in the context of the aforementioned diagnostic limitations. The challenge is that confounders remain, such as obesity, increased age, renal insufficiency, and anemia that may impact the diagnostic sensitivity and specificity of these traditional cardiac biomarkers [6,7]. There are several promising cardiovascular-based biomarkers including: high-sensitivity cardiac troponin I (cTn1), a myofibrillar protein released in the setting of supply/demand mismatch; interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), inflammatory markers associated with myocyte apoptosis and remodeling; endothelin-1 (ET-1), an acute-phase reactant that stimulates myocardial hypertrophy and cell injury; and pro-matrix metalloproteinase-9 (pMMP-9), a gelatinase subtype that contributes to left ventricular remodeling [7-13]. Whether these biomarkers can be incorporated routinely into standard clinical practice or if they are accurate prognosticators remains to be evaluated [14]. The objective of this cross-sectional study was to investigate the individual and combined role of biomarkers NT-proBNP, cTn1, IL-6, ET-1, pMMP-9, and TNF-α for risk stratification and prediction of HF severity and disease progression in patients with ACC/AHA stages A-D ischemic and non-ischemic cardiomyopathy (CM).
Study subjects
Two hundred patients with ACC/AHA Stages A-D were retrospectively identified from the Vanderbilt Heart and Vascular Institute (VHVI) Main Heart Registry and Biorepository. Written consent and plasma samples were obtained from each patient at time of enrollment following approval of the Vanderbilt Institutional Review Board. Past medical history, cardiovascular risk factors, demographic information, smoking history, ACC/AHA HF stage at time of enrollment, and medication history were collected. All patients who consented were included based on the following criteria: diagnosis of HF with varying severity, secondary to either ischemic or non-ischemic CM, with or without the presence of malignancy. Patients identified were followed from date of enrollment—considered to be baseline outpatient visit—and censored at the last date of follow-up. Information on dates of death, transplants, or implantation of left ventricular assist device (LVAD) were collected through electronic medical records or published obituaries. Baseline demographic data were collected using Vanderbilt University Medical Center electronic health records. Patients were excluded if they had received inotropic therapy during time of blood draw. Approximately 400 μL of EDTA plasma was obtained from frozen samples stored at -70°C. De-identified samples were then sent for analysis to Singulex Clinical Laboratory (Alameda, CA).
Biomarker assays
The research-use-only Erenna Immunoassay System, (Singulex, Inc., CA), powered by high-sensitivity single-molecule counting (SMC) technology, was used to measure the biomarkers cTnl, TNF-α, IL-6, ET-1, and pMMP-9 in EDTA plasma samples. Plasma samples, controls, capture and detection reagents, and standards were added to a 96-well plate and incubated. The aforementioned biomarkers of interest were bound to specific capture antibodies biotinylated to microparticles and fluorescently-conjugated detection antibodies. The unbound fluorescent antibodies were removed by a wash procedure, which was then followed by the addition of elution buffer to release fluorescent detection antibody into the eluent. The remaining contents of the 96-well plate were transferred to a 384-well plate and onto the Erenna system for SMC by counting photons passing through an interrogation space, which was directly proportional to the amount of biomarker within the sample. NTproBNP was measured on Roche cobas 6000 System using electrochemilluminescence immunoassay.
Statistical analysis
Descriptive statistics of baseline demographic and clinical characteristics were reported as continuous variables with median (interquartile range or IQR) and percentages for categorical variables. The cross-sectional associations of continuous biomarker measurements for NTproBNP, cTnI, ET-1, IL-6, TNF-α, and pMMP-9 across the four ACC/AHA HF stages were reported by expressing the median (IQR) for each biomarker at each HF stage. Post-hoc comparisons were then used to assess for statistically significant differences between HF stages and biomarker concentrations using Bonferroni family-wise error rate of α=0.05. Kaplan-Meier curves were generated to assess transplant- and advanced therapy-free survival with differences determined using the log-rank test dichotomizing cTnI and IL-6 at their median values. Additional comparisons were made between Cox Hazard Ratios that were 1) unadjusted, 2) adjusted by age and sex, 3) adjusted for ACC/AHA HF classification stages, and 4) adjusted for age, sex, race, estimated glomerular filtration rate, blood urea nitrogen, body mass index, systolic blood pressure, and ejection fraction to predict first incidence of death, heart transplant, or LVAD implantation.
Study subjects
A total of 200 patients within the Heart Failure cohort were enrolled and the demographics are listed in Supplemental Table 1. The plasma biomarker concentrations for all patients are detailed in Table 1 and highlighted in Figure 1. Baseline demographic information (median age 54 years) for patients within the Heart Failure cohort are presented in Supplemental Table 1. The majority of patients enrolled had a documented history of hypertension (71%), hyperlipidemia (54%), diabetes mellitus (66%). Forty-seven percent (47%) had a known history of tobacco use. Median baseline left ventricular ejection fraction (LVEF) was 30%. Median baseline creatinine was 1.1 pg/ml. Among the 200 patients identified retrospectively, 13% met criteria for Stage A HF, 22% Stage B, 33% Stage C, and 32% Stage D at time of enrollment.
| Table 1: Demographic information for heart failure cohort (n=200) | |
| Characteristic | Value |
| Age(years; median, IQR) | 54(44, 65) |
| Male(n, %) | 116(58%) |
| BMI(kg/m2, IQR) | 30.6(24.3, 33.6) |
| Hypertension(n, %) | 142(71%) |
| Hyperlipidemia(n, %) | 107(54%) |
| DiabetesMellitus (n, %) | 131(66%) |
| Smoking(n, %) | 93(47%) |
| BaselineLVEF (%; median, IQR) | 30(20, 55) |
| Creatinine(pg/ml; median, IQR)a | 1.1(0.9, 1.5) |
| ACC/AHAHF stage (n, %) | |
| A | 26(13%) |
| B | 43(22%) |
| C | 66(33%) |
| D | 65(32%) |
| BMI: Body mass index; LVEF: Leftventricular ejection fraction; ACC/AHA HF stage: American College ofCardiology/American Heart Association Heart Failure Stage; BMI: Body MassIndex; LVEF: Left Ventricular Ejection Fraction. | |
Plasma biomarkers measurements
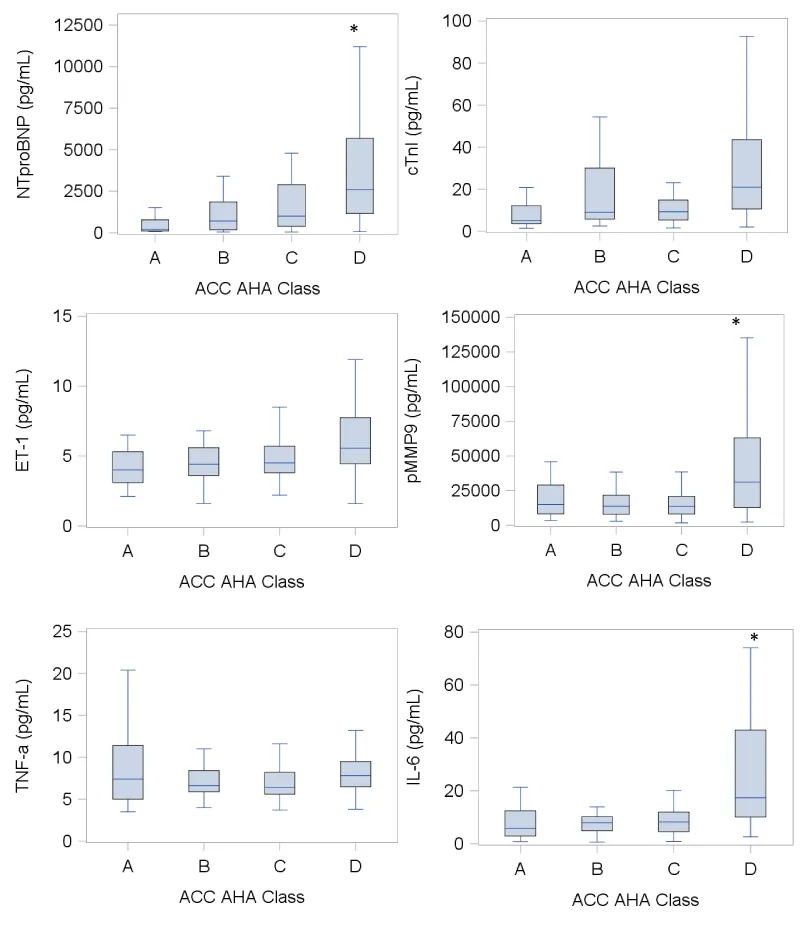
Continuous plasma concentrations of NTproBNP, cTnI, ET-1, pMMP-9, TNF-α, and IL-6 were measured across all ACC/AHA HF stages (Table 1). With the exception of TNF-α, there were significant correlations between HF severity and rising plasma concentrations of NTproBNP (p<0.0001), cTnI (p=0.0003), ET-1 (p<0.0001), pMMP-9 (p=0.0008), and IL-6 (p<0.0001). Additionally, post-hoc analyses were used to compare the plasma concentrations of the aforementioned biomarkers across varying stages of HF severity. Significant differences were observed between plasma concentrations of NTproBNP, pMMP-9, and IL-6 among individuals with ACC/AHA Class D HF in comparison to those measured among individuals with ACC/AHA Class A, B, and C HF (Figure 1). NTproBNP, cTnI, ET-1, pMMP-9, TNF-α, and IL-6 were also elevated during asymptomatic, early stages of HF.
Figure 1: Plasma biomarker measurements across all ACC/AHA HF stages. Box-and whisker plots of plasma biomarker concentrations with box plot boundaries representing 25th and 75th percentile, horizontal line denoting the median, whisker extending to 1.5x interquartile range; Statistical significance determined by Bonferroni post-hoc analyses to compare concentrations across HF groups; *p<0.05, denoting significant differences in plasma biomarker concentrations in stage D HF compared to stages A, B, and C for NTproBNP, pMMP-9, and IL-6; ACC/AHA, American College of Cardiology/American Heart Association; NTproBNP, N-terminal pro-Brain Natriuretic Peptide; cTnI, high sensitivity Troponin I; ET-1, Endothelin-1; pMMP-9, plasma Matrix Metalloproteinase-9; TNFα, Tumor Necrosis Factor-alpha; IL-6, Interleukin-6.
Survival analysis
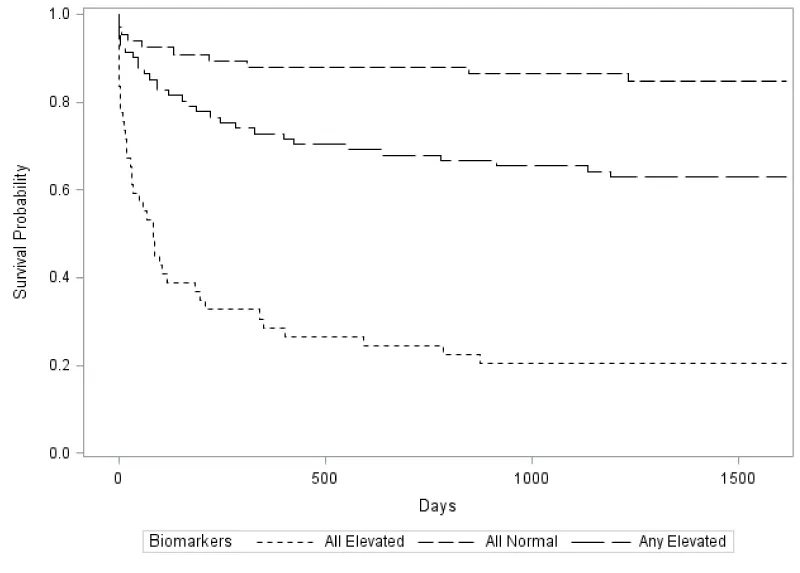
After 1500 days of follow-up, there were 35 reported deaths from all causes (17%), 36 cases of heart transplant (18%), and 27 cases of LVAD implantation (14%). Four patients were lost to follow-up. Higher circulating levels of NTproBNP, cTnI, and IL-6 predicted significantly worse survival for patients with HF at 1500 days based on comparisons of various combinations of those aforementioned plasma biomarker concentrations (Figure 2). Individuals were stratified using dichotomized median cutoff points into 3 groups: 1) NTproBNP >1144pg /mL + cTnI >11.3pg/mL + IL-6 >9.3pg/mL, 2) any one of the three biomarkers elevated and 3) NTproBNP <1144pg/mL + cTnI <11.3pg/mL + IL-6 <9.3pg/mL. Thus, combined elevations of NTproBNP, cTnI, and IL-6 demonstrated the greatest mortality risk compared to groups 2 and 3 (p<0.0001 for both). Additionally, Cox regression analysis adjusted for ACC/AHA classification demonstrated a significant difference in time to death, LVAD, or heart transplant with elevated IL-6 (HR 2.4, 95% CI 1.4-4) and cTnI (HR 2.0, 95% CI 1.2-3.3) in comparison to a model fully adjusted for age, sex, race, eGFR, BUN, BMI, baseline LVEF and systolic BP (Table 2).
Figure 2: Kaplan-Meier survival curves based on combined risk stratification for NTproBNP, cTnI, and IL-6 using dichotimized median cutoff points. Patients with elevated levels of all three biomarkers had significantly higher risk of adverse events compared to those with any one of the biomarkers elevated and those with biomarker concentrations below the median cutoff.
| Table 2: Measurements of plasma biomarkers across ACC/AHA HF stages. | ||||||
| ACC/AHA Class A(n=26) | ACC/AHA Class B(n=43) | ACC/AHA Class C(n=66) | ACC/AHA Class D (n=65) |
P value for trend | ||
| Biomarker (pg/mL) | NTproBNP | 216.0 (116.0, 667.0) |
723.0 (193.0, 1851.0) |
1007.0 (383.0, 2901.0) |
2613.0 (1165.0, 5675.0) |
<0.0001 |
| cTnI | 5.0 (3.7, 9.7) |
9.3 (5.8, 30.1) |
9.4 (5.4, 14.9) |
21.0 (10.6, 43.6) |
0.0003 | |
| ET-1 | 3.9 (3.1, 5.3) |
4.4 (3.6, 5.6) |
4.5 (3.8, 5.7) |
5.6 (4.5, 7.8) |
<0.0001 | |
| pMMP-9 | 15069.0 (8184.0, 27250.0) |
14287.0 (7982.0, 25894.0) |
13436 (8123.0, 20904.0) |
31139 (12819.0, 63088.0) |
0.0008 | |
| TNF-α | 7.3 (5.0, 10.6) |
6.6 (5.9, 8.7) |
6.4 (5.6, 8.2) |
7.8 (6.5, 9.5) |
0.3 | |
| IL-6 | 5.6 (2.9, 8.5) |
7.9 (4.9, 12.1) |
8.2 (4.6, 12.0) |
17.4 (10.1, 43.0) |
<0.0001 | |
| Continuous biomarker concentrationsdepicted as median (interquartile range Q1, Q3); ACC/AHA, American College ofCardiology/American Heart Association; NTproBNP, N-terminal pro-BrainNatriuretic Peptide; cTnI, high sensitivity Troponin I; ET-1, Endothelin-1;pMMP-9, plasma Matrix Metalloproteinase-9; TNF-a,Tumor Necrosis Factor-alpha; IL-6, Interleukin-6. | ||||||
This study examined the diagnostic and prognostic utility of several biomarkers among individuals with heart failure and left ventricular systolic dysfunction. We begin by demonstrating that NTproBNP, cTnI, IL-6, ET-1, and pMMP-9 are elevated in early, asymptomatic stages of HF. From this, we identified significant differences in plasma concentrations of NTproBNP, pMMP-9, and IL-6 between stage D and earlier HF stages, suggesting these might correlate with disease severity. With regards to prognostication, we observed that the combined effects of higher levels of NTproBNP, cTnI, and IL-6 were associated with worse survival compared to lower concentrations of these biomarkers. Additionally, when adjusted for ACC/AHA HF stages, there was a significant difference between IL-6 and cTnI concentrations with regards to time to death, LVAD, or heart transplantation.
In addition, we have shown that elevated IL-6 concentrations in combination with NTproBNP and cTnI predicted worse survival for patients with HF after a 1500-day follow-up period. This study confirms previously established findings regarding the diagnostic and prognostic utility of NTproBNP and cTnI. The BNP gene is activated in the setting of myocardial wall stress that ultimately leads to the processing of precursor proBNP into NTproBNP and BNP [30]. Increased levels of NTproBNP predict worse prognosis for HF and LVSD, and correspond with increased HF severity [30]. Although conventional assay systems have been less sensitive compared to BNP and NTproBNP kits, cTnI remains an important marker of survival and decompensation as it is also up-regulated during increased wall or oxidative stress [31]. We have demonstrated that rising levels of NTproBNP and cTnI were associated with increased HF severity, and that together with IL-6, predict worse survival in patients with HF and LVSD. This supports the role of IL-6 as an additive marker to aid in risk stratification and monitoring response to therapy. To our knowledge, this study is among the largest that confirms prior observations regarding the potential maladaptive role IL-6 plays in HF progression and mortality.
Regarding ET-1, we have shown that it is elevated during asymptomatic stages of HF. On a molecular level, ET-1 is an important mediator of cardiomyocyte hypertrophy through up-regulation of COX-2 resulting in an increase in cell surface area of rat neonatal cardiomyocytes and BNP mRNA synthesis, an effect that is augmented by its inter-dependency with angiotensin and norepinephrine [32,33]. In terms of its role in HF, ET-1 causes both afferent and efferent arteriole constriction, reducing renal blood flow and GFR [33]. ET-1 has also been shown to be an independent, short-term predictor of mortality in patients with heart failure with reduced ejection fraction (HFrEF) and acute heart failure [34,35]. These findings support the role of ET-1 as a potential diagnostic marker for HF during earlier stages, but the data for its role in risk stratification and prognostication is less compelling compared to the other biomarkers in this study (Table 3).
| Table 3: Comparison of ACC/AHA adjusted and fully adjusted hazard ratios with regards to time to death, LVAD, or heart transplantation among patients within the heart failure cohort. | ||||
| Biomarker | ACC/AHA Adjusted | Fully Adjusted | ||
| HR (95% CI) |
p value | HR (95% CI) |
p value | |
| NTproBNP | 1.5 (0.9-2.6) |
0.09 | 2.4 (1.4-4.4) |
0.003 |
| cTnI | 2.0 (1.2-3.3) |
0.007 | 2.6 (1.6-4.5) |
0.0003 |
| ET-1 | 1.5 (0.9-2.4) |
0.09 | 1.5 (0.9-2.4) |
0.1 |
| IL-6 | 2.4 (1.4-4.0) |
0.002 | 4.9 (2.8-8.7) |
<0.0001 |
| pMMP-9 | 1.2 (0.8-2.0) |
0.4 | 1.8 (1.1-2.9) |
0.02 |
| TNF-α | 1.2 (0.7-1.8) |
0.5 | 1.2 (0.7-1.9) |
0.5 |
| Hazard ratios adjusted for 1) ACC/AHA classification and 2) age, sex, race, baseline estimated glomerular filtration rate, blood urea nitrogen level, body mass index, baseline left ventricular ejection fraction, and baseline systolic blood pressure; ACC/AHA, American College of Cardiology/American Heart Association; NTproBNP, N-terminal pro-Brain Natriuretic Peptide; cTnI, high sensitivity Troponin I; ET-1, Endothelin-1; pMMP-9, plasma Matrix Metalloproteinase-9; TNF-a, Tumor Necrosis Factor-alpha; IL-6, Interleukin-6. | ||||
The diagnostic utility of pMMP-9 in the setting of heart failure and LVSD was also examined, with results suggesting that pMMP-9 is detectable in earlier stages of heart failure and correlates with HF severity. While pMMP-9 values independently predicted time to event in the fully adjusted model (HR 1.8; CI 1.1-2.9; p=0.02), we did not observe the same effect in the model adjusted for ACC/AHA HF stages. Up-regulation of MMP-9 contributes to ventricular remodeling through increased gelatinolytic activity that results in matrix degradation and myocardial collagen turnover [36,37]. However, epidemiologic studies have produced mixed results on the predictive utility of markers of collagen turnover such as MMP-9 in the setting of HF. Previous findings have demonstrated the former two observations showing that levels of pMMP-9 increased with increasing severity of NYHA class, and that higher pMMP-9 values were associated with higher probability of HF events alongside LV remodeling [38]. Yet, other studies have not observed such correlations between pMMP-9 levels, HF severity, and adverse outcomes, with another identifying it as a useful risk stratification marker in systolic HF only [39-41]. Additionally, when compared to BNP, pMMP-9 remained a poor clinical biomarker for ventricular remodeling and HF events [39]. Nonetheless, while its prognostic utility remains unclear, pMMP-9 may be a useful biomarker to measure alongside natriuretic peptides in order to evaluate for disease severity.
This study has several limitations. Several patients were lost to follow-up, and further adjustments could have been made during analysis to account for various causes of HF (i.e. ischemic vs. non-ischemic). However, other parameters were adjusted during survival analysis, including ACC/AHA HF stages, creatinine values, and LVEF. Furthermore, we have been able to retrospectively quantify on a relatively large scale a panel of diverse biomarkers in patients with HF and LVSD. As no interventions were performed, this limits the presence of confounding variables, which provided an assessment of the endogenous effects of these circulating biomarkers. Consequently, this has allowed for the investigation of both their diagnostic utility during early stages of HF, as well as prognosticating potential for patients with HF events despite optimal medication therapy.
These findings support the clinical applicability of NTproBNP, cTnI and IL-6 among patients with HF and LVSD for diagnostic and prognostic purposes, particularly when measured together. These biomarkers may also aid in risk stratification of asymptomatic individuals before symptoms of wall stress and volume overload become manifest, and these data could provide insight regarding preferred treatments to potentially prevent adverse outcomes. Future studies involving precision medicine are needed to examine and compare the individual and combined effects of multiple biomarkers on early detection and response to HF treatment based on individual profiles and development of targeted therapies in order to optimize HF management.
Conflicts of interest
DJL is a consultant for Pfizer, Roche, and Prothena, none of which are relevant to this study. Singulex employees were employed by Singulex Clinical Laboratory at the time of this study. All other authors report no conflicts of interest.
We would like to thank Dr. Johanna Sandlund (Singulex) for additional comments on the manuscript. This study was funded in part by Singulex Clinical Laboratory.
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016; 13: 368-378. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26935038
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation. 2016; 133: e38-360. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26673558
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128: e240-327. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23741058
- Khunti K, Baker R, Grimshaw G. Diagnosis of patients with chronic heart failure in primary care: usefulness of history, examination, and investigations. Br J Gen Pract. 2000; 50: 50-54. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10695070
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. Jama. 1989; 261: 884-888. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2913385
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017; 70: 776-803. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28461007
- Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2017; 135: e1054-e1091. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28446515
- Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010; 55: 2129-2137. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20447537
- Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006; 113: 2089-2096. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16636176
- Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002; 90: 520-530. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11909815
- Milo-Cotter O, Cotter-Davison B, Lombardi C, Sun H, Bettari L, et al. Neurohormonal activation in acute heart failure: results from VERITAS. Cardiology. 2011; 119: 96-105. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21912122
- Tsutamoto T, Wada A, Hayashi M, Tsutsui T, Maeda K, et al. Relationship between transcardiac gradient of endothelin-1 and left ventricular remodelling in patients with first anterior myocardial infarction. Eur Heart J. 2003; 24: 346-355. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12581682
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000; 106: 55-62. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10880048
- Gaggin H, Januzzi J. Cardiac biomarkers and heart failure. J Am Coll Cardiol. 2015.
- Jackson K, Rao V, Hanberg J, et al. Inflammation and Cardio-Renal Interactions in Heart Failure: A Potential Role for Interleukin-6. Journal of Cardiac Failure. 2017; 23: S25.
- Chen O, Patel J, Mohamed E, Greene M, Moskovits N, et al. Immunoregulatory role of cytokines in congestive heart failure. Microinflammation. 2014; 1.
- Fedacko J, Singh RB, Gupta A, Hristova K, Toda E, et al. Inflammatory mediators in chronic heart failure in North India. Acta Cardiol. 2014; 69: 391-398. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25181914
- Pudil R, Tichy M, Andrys C, Rehácek V, Bláha V, et al. Plasma interleukin-6 level is associated with NT-proBNP level and predicts short- and long-term mortality in patients with acute heart failure. Acta Medica (Hradec Kralove). 2010; 53: 225-228. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21400981
- Hamzic-Mehmedbasic A. Inflammatory Cytokines as Risk Factors for Mortality After Acute Cardiac Events. Med Arch. 2016; 70: 252-255. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27703283
- Lassus JP, Harjola VP, Peuhkurinen K, Sund R, Mebazaa A, et al. Cystatin C, NT-proBNP, and inflammatory markers in acute heart failure: insights into the cardiorenal syndrome. Biomarkers. 2011; 16: 302-310. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21417622
- Orus J, Roig E, Perez-Villa F, Paré C, Azqueta M, et al. Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant. 2000; 19: 419-425. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10808148
- Miettinen KH, Lassus J, Harjola VP, Siirilä-Waris K, Melin J, et al. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008; 10: 396-403. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18353715
- Pan JP, Liu TY, Chiang SC, Lin YK, Chou CY, et al. The value of plasma levels of tumor necrosis factor-alpha and interleukin-6 in predicting the severity and prognosis in patients with congestive heart failure. J Chin Med Assoc. 2004; 67: 222-228. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15357108
- Jug B, Salobir BG, Vene N, Sebestjen M, Sabovic M, et al. Interleukin-6 is a stronger prognostic predictor than high-sensitive C-reactive protein in patients with chronic stable heart failure. Heart Vessels. 2009; 24: 271-276. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19626399
- Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000; 36: 1587-1593. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11079662
- Marcucci R, Gori AM, Giannotti F, Baldi M, Verdiani V, et al. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. J Thromb Haemost. 2006; 4: 1017-1022. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16689753
- Chin BS, Blann AD, Gibbs CR, Chung NA, Conway DG, et al. Prognostic value of interleukin-6, plasma viscosity, fibrinogen, von Willebrand factor, tissue factor and vascular endothelial growth factor levels in congestive heart failure. Eur J Clin Invest. 2003; 33: 941-948. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14636296
- Eskandari V, Amirzargar AA, Mahmoudi MJ, Rahnemoon Z, Rahmani F, et al. Gene expression and levels of IL-6 and TNFalpha in PBMCs correlate with severity and functional class in patients with chronic heart failure. Ir J Med Sci. 2018; 187: 359-368. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28889349
- Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001; 103: 2055-2059. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11319194
- Kim HN, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation. 2011; 123: 2015-2019. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21555724
- Kawahara C, Tsutamoto T, Sakai H, Nishiyama K, Yamaji M, et al. Prognostic value of serial measurements of highly sensitive cardiac troponin I in stable outpatients with nonischemic chronic heart failure. Am Heart J. 2011; 162: 639-645. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21982655
- Li H, Gao S, Ye J, Feng X, Cai Y, et al. COX-2 is involved in ET-1-induced hypertrophy of neonatal rat cardiomyocytes: role of NFATc3. Mol Cell Endocrinol. 2014; 382: 998-1006. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24291639
- Rehsia NS, Dhalla NS. Potential of endothelin-1 and vasopressin antagonists for the treatment of congestive heart failure. Heart Fail Rev. 2010; 15: 85-101. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19763821
- Gottlieb SS, Harris K, Todd J, Estis J, Christenson RH, et al. Prognostic significance of active and modified forms of endothelin 1 in patients with heart failure with reduced ejection fraction. Clin Biochem. 2015; 48: 292-296. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25541019
- Perez AL, Grodin JL, Wu Y, Hernandez AF, Butler J, et al. Increased mortality with elevated plasma endothelin-1 in acute heart failure: an ASCEND-HF biomarker substudy. Eur J Heart Fail. 2016; 18: 290-297. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26663359
- Flevari P, Theodorakis G, Leftheriotis D, Kroupis C, Kolokathis F, et al. Serum markers of deranged myocardial collagen turnover: their relation to malignant ventricular arrhythmias in cardioverter-defibrillator recipients with heart failure. Am Heart J. 2012; 164: 530-537. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23067911
- Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998; 98: 1728-1734. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9788826
- Morishita T, Uzui H, Mitsuke Y, Amaya N, Kaseno K, et al. Association between matrix metalloproteinase-9 and worsening heart failure events in patients with chronic heart failure. ESC Heart Fail. 2017; 4: 321-330. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28772055
- Vorovich EE, Chuai S, Li M, Averna J, Marwin V, et al. Comparison of matrix metalloproteinase 9 and brain natriuretic peptide as clinical biomarkers in chronic heart failure. Am Heart J. 2008; 155: 992-997. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18513509
- Sanchis L, Andrea R, Falces C, Llopis J, Morales-Ruiz M, et al. Prognosis of new-onset heart failure outpatients and collagen biomarkers. Eur J Clin Invest. 2015; 45: 842-849. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26077878
- Buralli S, Dini FL, Ballo P, Conti U, Fontanive P, et al. Circulating matrix metalloproteinase-3 and metalloproteinase-9 and tissue Doppler measures of diastolic dysfunction to risk stratify patients with systolic heart failure. Am J Cardiol. 2010; 105: 853-856. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20211331