More Information
Submitted: 01 October 2019 | Approved: 16 October 2019 | Published: 17 October 2019
How to cite this article: Uysal BB, Akbas F, Altunoglu E, Denız GI, Uysal D, et al. The effect of anemia on serum hepcidin levels in patients with heart failure. J Cardiol Cardiovasc Med. 2019; 4: 159-163.
DOI: 10.29328/journal.jccm.1001059
ORCiD: 0000-0001-9292-5024
Copyright License: © 2019 Uysal BB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hepcidin; Heart failure
The effect of anemia on serum hepcidin levels in patients with heart failure
Betul Borku Uysal1*, Feray Akbas2, Esma Altunoglu2, Gulhan Ipek Denız3, Duygu Uysal2, Harun Uysal4, Hanife Usta Atmaca2, Yasin Yuksel5, Hale Aral6, Guven Cetın7, Cem Ar M8 and Fusun Erdenen2
1MD, Internal Medicine, Biruni University, Istanbul, Turkey
2MD, Internal Medicine, Istanbul Training and Research Hospital, Istanbul, Turkey
3Internal Medicine, Oncology, Sisli Hamidiye Etfal Training and Research Hospital Istanbul, Turkey
4Anesthesiology and Reanimation, Bezmialem University, Istanbul, Turkey
5Cardiology, Istanbul Training and Research Hospital, Istanbul, Turkey
6Biochemistry, Istanbul Training and Research Hospital, Istanbul, Turkey
7Internal Medicine, Hematology, Bezmialem University, Istanbul, Turkey
8Internal Medicine, Hematology, Cerrahpasa Faculty of Medicine Istanbul, Turkey
*Address for Correspondence: Betul Borku Uysal, MD, Assistant Professor, Internal Medicine, Biruni University, Istanbul, Turkey, Tel: +90505 6750677; Email: [email protected]
Background: Anemia is an accelerating problem among patients with heart failure (HF) and its presence is associated with more symptoms. In this study, we investigated whether anemia in heart failure was related to hepcidin concentration.
Methods: 50 patients with heart failure and 20 healthy subjects with no history of a chronic illness including heart failure as control group, were included in the study. Heart failure was verified by echocardiography in each subject and patients were defined as ones with reduced ejection fraction (HFrEF) if EF ≤ 40% and with preserved ejection fraction (HFpEF) if EF 40% - 50%. Blood samples were taken from all patients after 10-12 hours fasting. Anemia assessment was performed according to World Health Organization (WHO) criterias.
Results: There was a positive correlation between hepcidin concentration and urea, ferritin, hemoglobin, hematocrite, C-reactive protein (p < 0,05). Hepcidin concentrations of anemic heart failure patients were significantly lower than the non-anemic heart failure patients (p < 0,05).
Conclusion: We found that serum hepcidin concentration in anemic patients with heart failure was lower than in heart failure patients without anemia. We believe that iron defiency occurs as a result of inflammatory process in heart failure and therefore hepcidin concentrations decrease as a response. However, long-term follow up studies are needed.
Anemia is an accelerating problem among patients with heart failure [1,2]. Anemia is present in one-third of patients with heart failure and the presence of anemia in patients with heart failure is associated with increased symptoms, hospitalization and mortality [3]. The etiology of anemia in heart failure is multifactorial. The main question is whether anemia is a therapeutic target or just a marker of disease severity [3]. In recent years, “hepcidin”, a hormone discovered in peptide structure, has been described as the homeostatic regulator of iron absorption by allowing iron absorption from the intestine and macrophages and release of iron from hepatic stores.
Hepcidin synthesis stimulated during inflammation, improving the handling of iron from macrophages, by reduced plasma iron concentrations and inflammation leads to anemia. In this study, we investigated whether anemia in heart failure was related to hepcidin concentration.
Fifty patients with heart failure admitted to our clinic were included in our study. Inclusion criteria were being over 18 years old and having a history of heart failure longer than 6 months. Exclusion criteria included presence of kidney failure (serum creatinine level > 1.4), history of hemorrhage (gastrointestinal or severe menstrual bleeding), chronic liver disease (ALT > 2 folds), hematologic disease, iron overload diseases like hereditary hemochromatosis, iron replacement therapy or blood transfusion in the past six months, hypothyroidism, autoimmune disease and manifest infection. According to WHO criteria, the anemia diagnosis was made considering the hemoglobin value < 12 g/dl in women and < 13 g/dl in men. Heart failure was verified by echocardiography in each subject and patients were defined as ones with reduced ejection fraction (HFrEF) if EF ≤ 40% and with preserved ejection fraction (HFpEF) if EF 40% - 50%. 20 healthy subjects with no history of a chronic illness including heart failure were taken as control group.
A total of 70 patients, 50 of whom were heart failure patients and 20 control patients, were evaluated for age, sex and body weight. Blood samples were taken from all patients after 10-12 hours fasting. Complete blood count, urea, creatinine, iron, total iron binding capacity (TIBC), ferritin, vitamin B12, folate and sedimentation rate were determined. An extra tube of blood was taken from all patients for hepcidin kit. BD vacutein tubes were used in blood collection process. Samples were centrifuged at 4000 rpm (1600 g) and frozen at -80 °C in our biochemistry laboratory. Then all samples were determined by ELISA.
Ethics Committee informed on all matters relating to the study and our study protocol was approved by the Ethics Committee. Our study complies with the Declaration of Helsinki principles.
In the descriptive statistics of the data mean, standard deviation, mime-maker media, ratio and frequency values were used. The distribution of the variables was checked by the Kolmogorov-Smirnov test. Independent sample t test and Mann-Whitney U test were used in the analysis of qualitative data. The Chi-square test was used to analyze qualitative data, and the Fischer test was used when chi-square test conditions were not met. Pearson, Spearman correlation analysis was used for correlation analysis. The significance level of the obtained results is interpreted as “p” value. p < 0.05 was considered statistically significant. SPSS 21.0 program was used in the analysis.
Thirty-three (66%) of the cases were male and 17 (34%) were female. Six of the control group (30%) were male and 14 (70%) were female. The rate of male patients in the case group was significantly higher than that in the control group (p < 0.05). In the case group, the mean age of the patients was 62.5 ± 10.5 years and the mean age in the control group was 51.7 ± 9.2 years. The age of the patients in the case group was significantly higher than the control group (p < 0.05). The mean weight of the patients in the case group was 73.7 ± 8.0, and the mean weight in the control group was 68.4 ± 10.3. Patients’ weight in the case group was significantly higher than control group (p < 0.05).
There was no correlation between hepcidin concentration and age, weight, creatinine, iron, vitamin B12, folate, white blood cell (WBC), platetelet, mean corpuscular volume, sedimentation rate, ejection fraction (EF) (p > 0,05). There was a positive correlation between hepcidin concentration and urea, ferritin, hemoglobin, hematocrite, C-reactive protein (p < 0,05).
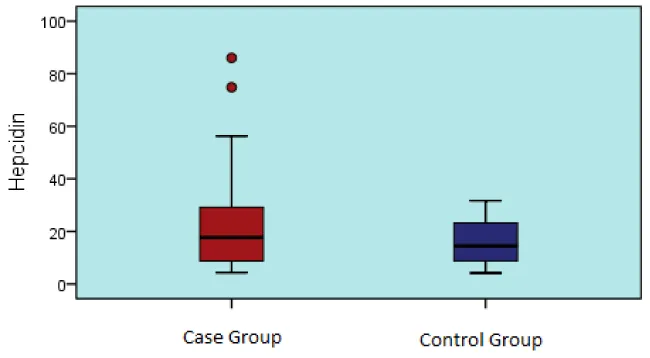
In the case and control group, hepcidin value was not significantly different (p > 0.05) (Table 1, Graphic 1).
| Table 1: Hepcidin values in case and control groups. | ||||||||||
| Case Group | Control Group | |||||||||
| Mean ± s.s./n - % | Median(min - max) | Mean ± s.s. | Median (min - max) | p | ||||||
| EF | 36,9 ± 6,9 | 38 | 15 - 55 | |||||||
| < 40% | 27 | 54,0% | ||||||||
| EF | 40% - 50% | 23 | 46,0% | |||||||
| Hepcidin | 21,5 ± 17,4 | 18 | 4 – 86 | 16,3 ± 8,5 | 15 | 4 - 32 | 0,209t | |||
| tIndependent sample t test | ||||||||||
Graphic 1: Hepcidin values in case and control groups.
EF values were not significantly different (p > 0.05) in the anemic and non-anemic groups (Table 2).
| Table 2: Hepcidin levels in anemic and non-anemic heart failure patients. | |||||||||||
| Anemic | Non-Anemic | ||||||||||
| Mean ± s.s. | Median (min-max) | Mean ± s.s. | Median (min-max) | p | |||||||
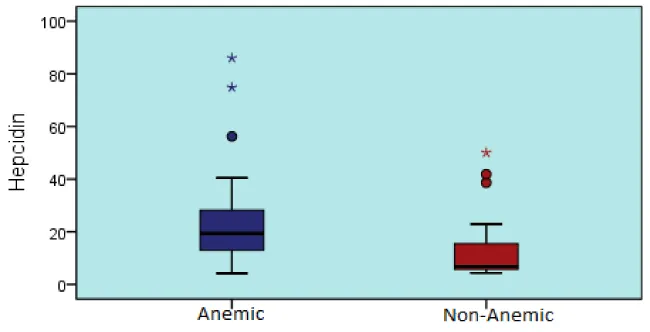
| Hepcidin | 22,6 | 15,8 | 19 | 4 | 86 | 13,9 | 13,4 | 7 | 4 | 50 | 0,030t |
| EF | 37,0 | 7,1 | 38 | 20 | 55 | 36,6 | 6,8 | 40 | 15 | 44 | 0,877t |
| tIndependent sample t test | |||||||||||
Hepcidin concentrations of anemic heart failure patients were significantly lower than the non-anemic heart failure patients (p < 0,05) (Table 2, Graphic 2).
Graphic 2: Hepcidin levels in anemic and non-anemic heart failure patients.
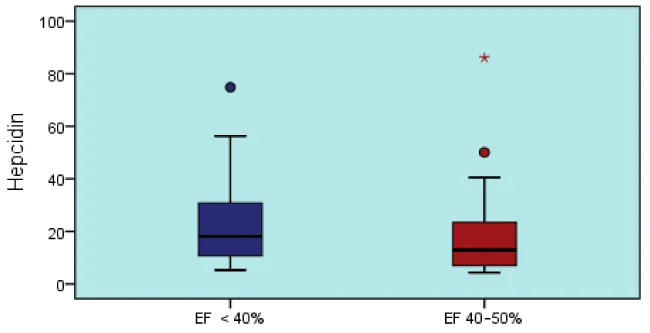
Patients with EF values < 40% and 40% - 50% did not differ significantly (p > 0.05) (Table 3, Graphic 3).
| Table 3: Hepcidin level changes according to EF levels. | |||||||
| EF < 40% | EF 40% - 50% | ||||||
| Mean ± s.s. | Median (min - max) | Mean ± s.s. | Median (min - max) | p | |||
| Hepcidin | 22,9 ± 16 | 18 | 5 - 74,8 | 19,8 ± 19 | 13 | 4 - 86 | 0,545t |
| tIndependent sample t test | |||||||
Graphic 3: Hepcidin level changes according to EF levels.
Heart failure is a complex clinical syndrome resulting from the inability of the heart to pump blood, due to any structural or functional heart disease that disrupts the ventricular filling and / or blood pumping ability, to meet the metabolic needs of the tissues [1,2,5]. Heart failure, as in all chronic diseases is frequently associated with anemia. Approximately one-third of patients with heart failure are anemic [3]. This leads to worsening symptoms of heart failure, diminished quality of life, increased hospitalization rate and reduced survival. The etiology of anemia in patients with HF is thought to be due to many factors [6,7], such as chronic inflammation [8], hemodilution [9], renal dysfunction [10], resistance to erythropoietin [11], hemolysis [12], bone marrow dysfunction [13], hematinic shortcomings including vitamin B12, folic acid and iron deficiency [14].
In our study, the mean age was 62.5 ± 10.5 in the case group and the mean age in the control group was 51.7 ± 9.2. So in the case group than in control group age of the patients significantly (p < 0.05) were higher. The most important reason for this is that heart failure is more common in the older population [1,15]. In general, the prevalence of HF is around 2% in the 40-59 age group, while about 5% in the 60-69 age group is approximately 10% in the age group of 70 years [1]. In younger age groups, the prevalence of males and females is equal in males, and is even more common in females after the age of 80. While it is more common among males in the young age group, the proportion of males and females in the middle aged is equalized, and is more common in females in older ages. In our study, the rate of male patients was higher in the case group.
The weight of the patients in the case group was significantly higher. We attributed this situation to weight gain due to increased weight due to congestion in heart failure and decrease in exercise capacity.
One of the exclusion criteria of our study was the known renal failure patients. We also preferred patients with a creatinine value of < 1.4 to exclude unknown renal failure. However, urea and creatinine concentrations were found to be significantly higher in patients with heart failure than normal controls. The most important reason for this is cardiorenal syndrome. Ronco, et al. [16], in their study revealed that there was a communication between the heart and kidney as two aspects and classified the cardiorenal syndrome. According to this study, sympathetic, neurohumoral and inflammatory mediators are released as a result of insufficiency in the heart and vasoconstriction in the kidney. This situation causes a decrease in glomerular filtration rate in long term. In our patients, urea and creatinine concentrations, which were found to be higher in the normal range than in the control group, could be considered as a predictor of renal failure, which may develop in patients, even if they did not produce a clear renal failure.
Matsumoto, et al. [17], found that serum iron and transferrin saturation in heart failure patients with anemia were found to be within the normal range but significantly lower than control group. In our study, iron concentrations of patients with heart failure were significantly lower and iron binding capacity was significantly higher than control group. However, bone marrow biopsy should be evaluated to determine the actual iron concentration in the body and this may be considered as a limitation of our study.
We also evaluated vitamin B12 and folic acid concentrations for other etiologies of anemia. However, no significant difference was found between the case and control groups in terms of vitamin B12 and folate concentrations.
Heart failure is often a chronic disease associated with anemia. Many factors determine the etiology and pathogenesis of anemia in patients with heart failure. These include hemodilution, loss of iron due to anti-platelet drugs, inflammatory cascade activation and loss of erythropetine from renal failure [18]. In a previous study, Adlbrecht, et al. [19], described the cause of anemia in heart failure as a relative increase in plasma volume rather than a massive reduction in red blood cell. However, the study did not use a control group without heart failure. We did not measure the plasma volume and red blood cell mass. This is a limitation for our study.
Matsumoto, et al. [17] examined serum hepcidin concentrations in patients with heart failure. 36 heart failure patients with anemia, 16 patients without heart failure and anemia were compared. They found that serum hepcidin concentrations in heart failure patients with anemia were lower than those in other groups and that the anemia of inflammation was a minor criteria in the development of anemia in heart failure patients. In a subsequent study [20]. Divakaran and colleagues evaluated a total of 97 patients and examined both urine and serum hepcidin concentrations in patients. Compared with the control group, they found urinary and serum hepcidin concentrations lower in heart failure patients with anemia. They did not find any difference between urine and serum hepcidin concentrations of the heart failure patients with or without anemia. As a result, in support of Matsumoto, et al.’s conclusion, they concluded that hepcidin did not play a major role in the anemia that developed in patients with heart failure. Our results support Matsumoto, et al. and Divakaran, et al. According to our study, hepcidin value did not show any significant difference in patient and control groups. However, heart failure patients with anemia had significantly lower hepcidin concentrations than patients with heart failure without anemia. Jankowska, et al. [12], recently performed a 3-year mortality study of 387 patients, including 321 heart failure and 66 controls, and examined hepcidin concentrations. They stated that the elevated hepcidin value was indicative of early heart failure, and was not related to anemia and inflammation. They pointed out that there was a correlation between progressive heart failure and decreased serum hepcidin concentrations and development of iron deficiency. Low hepcidin concentrations were evaluated as independent negative results.
Ohno, et al. [7], and Suziki, et al. [6], found that if the liver congestion develops in heart failure, the hepcidin concentration increases then iron deficiency and anemia occurs accordingly. Recent studies have shown that intravenous iron replacement therapy is superior to oral iron replacement in anemia that develops in heart failure [21]. Even anti-hepcidin treatment studies in the anemia of heart failure have been discussed [22].
We found that serum hepcidin concentrations were lower in anemic patients with heart failure than in patients with heart failure without anemia. Previously, progression of heart failure was found to be associated with decline in circulating hepcidin and the development of iron deficiency and low hepcidin concentration was related to unfavorable out come.
We believe that primarily hepcidin increases as a result of an inflammatory process associated with heart failure. As a result, when iron absorption is reduced and heart failure progresses and when anemia develops, hepcidin concentrations decrease as a response. Therefore, in addition to anti-hepcidin therapies, efforts to develop anti-inflammatory treatments for cardiac insufficiency should be on the agenda. However, long-term follow up studies are needed.
- McMurray JJ. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012; 14: 803-869.
- Yancy CW. 2013 ACCF/AHA guideline for the management of heart failure. Circulation. 2013.
- Grote Beverborg N, van Veldhuisen DJ, van der Meer P. Anemia in Heart Failure: Still Relevant? JACC Heart Fail. 2018; 6: 201-208. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29128254
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003; 102: 783-788. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12663437
- Douglas L Mann. Harrison’s Principles of Internal Medicine, in 20th. 2018, McGraw Hill. 2018; 1763-1769.
- Suzuki T, Hanawa H, Jiao S, Ohno Y, Hayashi Y, et al., Inappropriate expression of hepcidin by liver congestion contributes to anemia and relative iron deficiency. J Card Fail. 2014; 20: 268-277. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24440572
- Ohno Y, Hanawa H, Jiao S, Hayashi Y, Yoshida K, et al., Liver congestion in heart failure contributes to inappropriately increased serum hepcidin despite anemia. Tohoku J Exp Med. 2015; 235: 69-79. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25742771
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005; 352: 1011-1023. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15758012
- Androne AS, Katz SD, Lund L, LaManca J, Hudaihed A, et al. Hemodilution is common in patients with advanced heart failure. Circulation. 2003; 107: 226-229. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12538419
- Eschbach JW. Anemia management in chronic kidney disease: role of factors affecting epoetin responsiveness. J Am Soc Nephrol. 2002; 13: 1412-1414. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11961032
- van der Meer P, Lok DJ, Januzzi JL, de la Porte PW, Lipsic E, et al. Adequacy of endogenous erythropoietin levels and mortality in anaemic heart failure patients. Eur Heart J. 2008; 29: 1510-1515. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18495690
- Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, et al. Iron status in patients with chronic heart failure. Eur Heart J. 2013; 34: 827-834. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23178646
- Westenbrink BD, Voors AA, de Boer RA, Schuringa JJ, Klinkenberg T, et al. Bone marrow dysfunction in chronic heart failure patients. Eur J Heart Fail. 2010; 12: 676-684. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20427614
- Witte KK, Desilva R, Chattopadhyay S, Ghosh J, Cleland JG, et al. Are hematinic deficiencies the cause of anemia in chronic heart failure? Am Heart J. 2004; 147: 924-930. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15131553
- Douglas L Mann, MC. Harrison’s Principles of Internal Medicine, in 19th. 2015, McGraw Hill. 1500-1507.
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008; 52: 1527-1539. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19007588
- Matsumoto M, Tsujino T, Lee-Kawabata M, Naito Y, Akahori H, et al., Iron regulatory hormone hepcidin decreases in chronic heart failure patients with anemia. Circ J. 2010; 74: 301-306. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20019408
- Sandhu A, Sandeep Soman, Michael Hudson, Anatole Besarab. Managing anemia in patients with chronic heart failure: what do we know? Vasc Health Risk Manag. 2010; 6: 237-252.
- Adlbrecht C, Kommata S, Hülsmann M, Szekeres T, Bieglmayer C, et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body's red cell volume. Eur Heart J. 2008; 29: 2343-2350. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18701467
- Divakaran V, Mehta S, Yao D, Hassan S, Simpson S, et al. Hepcidin in anemia of chronic heart failure. Am J Hematol. 2011; 86: 107-109. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21080339
- Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, et al., Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018; 20: 125-133. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28436136
- Cooke KS, Hinkle B, Salimi-Moosavi H, Foltz I, King C, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013; 122: 3054-3061. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23945155