More Information
Submitted: 15 October 2019 | Approved: 26 October 2019 | Published: 28 October 2019
How to cite this article: Abd El-Moneum MS. Evaluation of the predictive value of CHA2DS2-VASc Score for no-reflow phenomenon in patients with ST-segment elevation myocardial infarction who underwent Primary Percutaneous Coronary Intervention. J Cardiol Cardiovasc Med. 2019; 4: 171-176.
DOI: 10.29328/journal.jccm.1001061
ORCiD: 0000-0002-2459-3619
Copyright License: © 2019 Abd El-Moneum MS. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: ST-segment elevation myocardial infarction; Percutaneous coronary intervention; CHA2DS2-VASc score; No-reflow phenomenon
Evaluation of the predictive value of CHA2DS2-VASc Score for no-reflow phenomenon in patients with ST-segment elevation myocardial infarction who underwent Primary Percutaneous Coronary Intervention
Mahmoud Shawky Abd El-Moneum*
Cardiology Department, Faculty of Medicine, Benha University, Egypt
*Address for Correspondence: Betul Borku Uysal, MD, Assistant Professor, Internal Medicine, Biruni University, Istanbul, Turkey, Tel: +90505 6750677; Email: [email protected]
Objective: The aim of this study was to estimate the predictive clinical value of CHA2DS2-VASc score for no-reflow phenomena in patients having ST-segment elevation myocardial infarction (STEMI) who applied to primary percutaneous coronary intervention (PCI).
Subjects and Methods: Three-hundred STEMI patients underwent primary PCI. They were classified into: group (1) included 27 patients with no-reflow and group (2) included 273 patients without no-reflow (control). CHA2DS2-VASc risk score was computed for each patient.
Results: This study found statistically significant difference (p < 0.05) in multivariate analysis of the association between CHA2DS2-VASc score and no-reflow phenomenon. The predictive power of individual components in CHA2DS2-VASc score for no-reflow was statistically significant difference (p < 0.05). So, significantly higher CHA2DS2-VASc score is connected with higher risk of no-reflow and in-hospital mortality rate.
Conclusion: Significantly higher CHA2DS2-VASc score is associated with higher risk of no- reflow phenomenon and in-hospital mortality rates in patients with STEMI who underwent primary PCI.
In patients with STEMI, the reason of primary PCI is prompt return of typical blood stream within the infarct-related artery [1]. Nevertheless, no-reflow phenomenon could be a major challenging drawback of this method. No-reflow is characterized as insufficient myocardial perfusion in spite of mechanical reviving of the offender injury with PCI. This marvel is related to higher frequency of complications, and brief- and long-term mortality in STEMI patients [2].
No-reflow phenomenon happens in 0.6% to 5% of elective PCIs, but a higher frequency has been detailed in patients who experienced primary PCI [3]. A multifactorial and complex pathophysiology has been proposed for component of this phenomenon [4]. Tragically, there’s no broadly acknowledged hazard stratification strategy for the forecast of this complication [5].
The no-reflow phenomenon happens since of heterogeneous components counting distal embolization of flotsam and jetsam relating to ulcerated atherosclerotic plaques, microvascular harm, vasospasm, insuperable of oxidative stretch, and reperfusion damage [6]. Thus, a dependable hazard stratification device is required, which legitimately predicts the rate of no-reflow with respect to its multifactorial pathogenesis [7].
CHA2DS2-VASc score is considered a clinical indicator of thromboembolic diseases and was suggested for anticoagulant treatment in patients with nonvalvular atrial fibrillation in clinical rules [8]. The components of this score are common hazard variables of atherosclerosis, microvascular injury as well as no-reflow and stroke. Moreover, utilize of this score is exceptionally basic and makes it a speedy apparatus to predict no-reflow phenomenon, comparative to common chance variables of the no-reflow [5].
Aim of the work
The aim of this study was to evaluate CHA2DS2-VASc score in predicting no-reflow phenomenon and in-hospital mortality in patients with STEMI who underwent primary PCI.
Study design and population
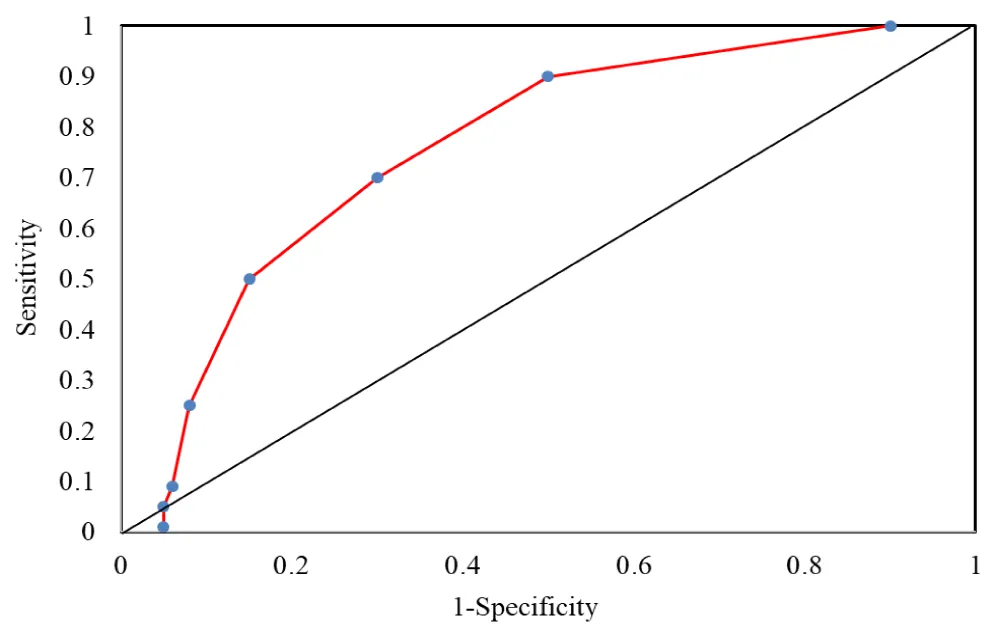
This study was performed over a period of two years from August 2017 to August 2019 and it was conducted in Cardiology Department, Faculty of Medicine, Benha University, Egypt on three-hundred (300) STEMI patients who underwent PCI. They were classified into two main groups; group (1) included 27 patients with no-reflow complication after PCI and group (2) included 273 patients without no-reflow used as control group figure 1.
Figure 1: AUC (Area under the curve) of CHA2DS2-VASc score = 2 in predicting no-reflow.
Inclusion criteria included patients with STEMI referred for primary PCI after diagnostic coronary angiography.
Exclusion criteria included patients either with a delayed diagnosis of STEMI or deferred arrival whose symptoms persisted longer than 12h. Furthermore, subjects who advanced non-significant stenosis in the culprit vessel or whom their coronary anatomy was not eligible to perform PCI. Patients with favorable criteria for coronary artery bypass grafting (CABG), stenosis of the saphenous vein grafts (SVGs) as culprit lesions, and coronary artery dissection were also ruled out. The Ethics Committee of the hospital approved the protocol and informed consent was obtained in every subject.
All participants included in this study were subjected to:
- Informed consent: each participant has to perform written consent
- Full history taking: including family history, history of hypertension, diabetes mellitus and smoking habit, onset and duration of the disease.
- General and local examination of the heart.
- 12 leads ECG
- Routine laboratory tests including: complete blood picture, liver functions, renal functions, serum calcium and lipid profile.
- Conventional echocardiography was done for all patients.
- Coronary angiography & primary PCI were performed for all patients.
Diagnosis of STEMI
STEMI diagnosis was verified according to a history of a typical chest pain accompanied with ST-segment elevation at the J point of 1 mm or more in two contiguous leads at least with the following cut points.
ST elevation ≥ 0.1 mV in all leads (except V2-V3). In leads, V2 and V3 these cut off values apply: ≥ 0.2 mV for men ≥ 40 years, ≥ 0.25 mV in men under 40 years, ≥ 0.15 mV in women. Furthermore, new left bundle branch block (LBBB) [10].
TIMI grading
The thrombolysis in myocardial infarction (TIMI) stream was decided after primary PCI, comprised of four grades: TIMI-0 shows that there’s no forward coronary stream in angiography post the location of stenosis or impediment, TIMI-1 depicts a destitute distal antegrade stream driving to deficient filling of the supply route, TIMI-2 illustrates a conceded moderate frontward stream that fills the distal region totally, TIMI-3 delineates typical coronary stream [6].
CHA2DS2-VASc score
CHA2DS2-VASc risk score was computed for each patient based on the definition proposed by Lip, et al. [8]. This risk assessment tool is a grouped subtending eight components with specified scores. The acronym represents as heart failure (C), hypertension (H), age ≥ 75 years (A2), diabetes mellitus (D), stroke (S2), vascular disease (V), age 65 to 74 years (A), and female gender (as a sex category). Quantified values pertaining to stroke and Age over 75 are determined with two points while 1 point was assigned to each of the remaining variables [8]. The cutoff value of CHA2DS2-VASc risk score ≥ 2 was believed as a predictor of no- reflow with a sensitivity of 66% and a specificity of 59%.
Myocardial blush grades
Myocardial blush grades (MBG) were quantified based on the previous classifications. Absence of myocardial blush or contrast density means Grade 0. Grade 1 refers to least contrast density. Grade 2 is known as a remark of moderate myocardial blush but less than that reported during an angiography of an ipsilateral or contralateral non-infarct-related coronary artery, and Grade 3 shows “normal myocardial blush or contrast density in compare with that obtained throughout an angiography of an ipsilateral non-infarct-related or contralateral coronary artery” [11]. We revised angiography sine films to evaluate no-reflow/slow flow occurrence using a combination of TIMI flow and MBG. Occurrence of TIMI flow < 3 with any MBG grade or TIMI flow 3 attended with MBG 0 or 1 were considered as suboptimal reperfusion while successful reperfusion was defined as TIMI flow 3 with MBG 2 or 3.
Statistical analysis
Persistent factors were communicated as cruel ± standard deviation whereas categorical factors were appeared by rates. Two-tailed Student’s t-test and Mann–Whitney U test were enlisted to compare ceaseless factors with and without typical dispersion, individually. Chi-square test was utilized to appear the distinction of categorical factors. We have moreover illustrated separated and complex interaction-mediated impacts of indicators of no-reflow marvel. In this manner, univariate and multivariate relapse examinations were performed to assess unadjusted and balanced affiliation of potential chance components extraordinarily CHA2DS2-VASc and imperfect coronary stream. We decided the prescient utility of the CHA2DS2-VASc score for ensuing results counting no-reflow and in-hospital mortality utilizing collector working characteristic bends. Measurable importance was affirmed with a p - value < 0.05. All investigations were conducted utilizing SPSS form 22 (SPSS Inc., Chicago, IL, USA).
The study was based on comparison between patients of group (1), 27 patients with no-reflow phenomenon and group (2) that includes 273 patients without no-reflow complications in STEMI patients who undergone primary PCI (Table 1).
| Table 1: Age and sex distribution of the studied groups. | ||||||
| Variable | Group (1) (N = 27) |
Group (2) (N = 273) |
Test of significance | |||
| t - test | p - value | |||||
| Age (years): | 59 – 84 68.7 ± 14.2 |
52 – 69 59.8 ± 13.9 |
3.92 | 0.011* | ||
|
||||||
| Sex: | N | % | N | % | χ2-test | p - value |
|
9 18 |
33.3 66.7 |
150 123 |
54.9 45.1 |
-3.216 4.109 |
0.0092* 0.0021* |
| p < 0.05 = non-significant. | ||||||
Assessment of cardiovascular risk factors, clinical, laboratory & echocardiographic Characteristics of the patients:
Hypertension, diabetes mellitus, anemia, Body Mass Index (BMI), serum creatinine, left ventricular ejection fraction (LVEF), systolic blood pressure (SBP), diastolic blood pressure (DBP), calcium scoring, anterior MI, vascular disease and in- hospital mortality showed a statistically significant difference (p < 0.05) between the two groups as presented in table 2.
| Table 2: Risk factors , clinical, laboratory & echocardiographic Characteristics of the patients | ||||||
| Risk factors | Group (1) (N = 27) |
Group (2) (N = 273) |
Statistical test of significance | |||
| N | % | N | % | χ2 - test | p - value | |
| Hypertension | 27 | 100 | 253 | 92.7 | 2.544 | 0.029* |
| Diabetes mellitus | 27 | 100 | 267 | 97.8 | 1.846 | 0.043* |
| Dyslipidemia | 27 | 100 | 270 | 98.9 | 2.629 | 0.171 |
| Anemia | 20 | 47.1 | 105 | 38.5 | 3.012 | 0.006* |
| Smoking | 21 | 77.8 | 210 | 76 | 3.695 | 0.182 |
| Family history | 16 | 59.3 | 164 | 60.7 | 0.098 | 0.182 |
BMI (Kg/m2):
|
8 19 |
29.6 70.4 |
98 175 |
35.9 64.1 |
-2.973 3.111 |
0.037* 0.013* |
| Vascular disease | 7 | 25.2 | 37 | 13.7 | 3.112 | 0.001* |
| MI type, anterior | 16 | 59.2 | 121 | 44.3 | 3.112 | 0.001* |
| In- hospital mortality | 3 | 11.1 | 7 | 2.56 | 6.991 | 0.001* |
Calcium:
|
2 25 |
7.407 92.59 |
86 187 |
31.41 68.49 |
-8.466 6.557 |
0.000* 0.001* |
| Mean ± SD | Mean ± SD | t-test | p - value | |||
| Creatinine | 1.08 ± 0.62 | 0.94 ± 0.52 | 3.969 | 0.018* | ||
| LV Ejection fraction (%) | 37.8 ± 10.9 | 42.7 ± 9.98 | 9.25 | 0.000* | ||
| Systolic blood pressure | 149.5 ± 22.8 | 136.8 ± 21.9 | 1.925 | 0.041* | ||
| Diastolic blood pressure | 85.6 ± 16.8 | 76.3 ± 15.7 | 1.728 | 0.044* | ||
| *p < 0.05 is a statistically significant value. BMI: body mass index. | ||||||
PCI characteristics
Higher Stent length & lower stent diameter were associated with no-reflow phenomenon (p < 0.05) as found in table 3.
| Table 3: Characteristics of PCI | ||||||
| Variable | Group (1) (N = 27) |
Group (2) (N = 273) |
Test of significance | |||
| t - test | p - value | |||||
| Stent length (mm) | 28.1 ± 6.8 | 24.2 ± 7.5 | 2.62 | 0.017* | ||
| Stent diameter (mm) | 2.79 ± 0.52 | 3.12 ± 0.49 | 2.56 | 0.018* | ||
Location:
|
N | % | N | % | U-test | p - value |
| 16 3 8 |
59.3 11.1 29.6 |
136 28 109 |
49.8 10.3 39.9 |
1.652 | 0.062 | |
| * p < 0.05 is a statistically significant value. U: Mann-Whitney U-test. | ||||||
Evaluation of CHA2DS2-VASc score
Analysis of CHA2DS2-VASc score and its different components revealed that they were statistically significant in comparison between patients with no-reflow group and patients without no-reflow. In multivariate analysis (Table 3), there is statistically significant difference (p < 0.05) between the two groups as regard CHA2DS2-VASc score, BMI, hypertension, systolic ,diastolic blood pressure, and initial TIMI flow rate ≥ 1. In univariate analysis; there is statistically significant difference (p < 0.05) between the two groups as regard congestive heart failure, hypertension, old age, diabetes mellitus, transient ischemic attacks or stroke, vascular diseases and female gender as shown in tables 4,5.
| Table 4: Multivariate regression analysis of the association between CHA2DS2-VASc score and no-reflow phenomenon. | |||
| Predictor | OR | 95% CI | p - value |
| CHA2DS2-VASc score | 2.06 | 1.91 – 4.62 | 0.000* |
| BMI | 1.07 | 1.02 – 1.32 | 0.034* |
| Hypertension | 2.28 | 2.14 – 4.65 | 0.009* |
| SBP | 0.59 | 0.48 – 1.09 | 0.004* |
| DBP | 0.62 | 0.49 – 1.12 | 0.003* |
| Initial TIMI flow rate ≥1 | 0.48 | 0.38 – 0.62 | 0.001* |
| OR: Odds ratio, CI: confidence interval, p < 0.05 = significant, SBP: systolic blood pressure, BMI: body mass index, DBP: diastolic blood pressure, TIMI: thrombolysis in myocardial infarction. | |||
| Table 5: Univariate regression analysis of predictive power of individual components in CHA2DS2-VASc score for no-reflow. | |||
| Variable | OR | 95% CI | p - value |
| Congestive heart failure | 21.2 | 8.11 – 63.5 | 0.000* |
| Hypertension | 4.95 | 3.55 – 9.85 | 0.001* |
| Age >75 years | 2.12 | 1.02 – 3.97 | 0.007* |
| Diabetes mellitus | 4.68 | 3.17 – 9.25 | 0.000* |
| Transient ischemic attack, stroke | 4.09 | 2.04 – 14.7 | 0.016* |
| Vascular disease | 7.59 | 5.01 – 14.2 | 0.001* |
| Gender (females) | 3.21 | 1.67 – 4.18 | 0.002* |
Primary PCI is the preferred revascularization method in most patients with a diagnosis of acute STEMI, but acute reduction in myocardial blood flow after this procedure despite a patent epicardial coronary artery, the so-called “no-reflow phenomenon”, leads to adverse outcomes in these patients [2]. Some studies suggest deferring stent strategy to reduce no-reflow after PCI [12].
Results of this study confirmed the clinical implication of CHA2DS2-VASc score that playing an extra role in prediction of contrary outcomes following primary PCI in STEMI patients. The use of risk score can discriminate STEMI patients at higher risk of no-reflow after PCI.
This study found that increased CHA2DS2-VASc score is an independent predictor of mortality rather than a surrogate measure only. This was in agreement with other studies [6-8].
There are multiple risk stratification models for assessment of prognosis after acute coronary syndrome. However, some models are complex or contain variables, which are not available at presentation data. Due to time restrictions that is determined for observation of STEMI revascularization, practical aids of a simple accustomed risk score such as CHA2DS2-VASc becomes more prominent [13].
This study revealed a dual prognostic utility of CHA2DS2-VASc for both suboptimal reperfusion and short-term mortality. This coincides with many studies who found a useful surrogate measure of in-hospital mortality independent of no-reflow [5,12,14-18].
Failure of reperfusion or no-reflow often connects with extended myocardial necrosis and also poor clinical outcome irrespective of infarct size [19,20].
Ndrepepa G, et al. [19], have reported that no-reflow elevated one-year attuned risk of death after primary PCI by 3-folds so, early identification of patients with STEMI vulnerable to no-reflow is important to prevent or minimize the risk of ineffective reperfusion.
Diabetes mellitus, as a constituent of CHA2DS2-VASc score, impairs normal endothelial function and perpetuates ischemic reperfusion injury [3,21]. In contrast to these findings Iwakura, et al. [22] did not find diabetes mellitus as a predictor despite demonstrating an association between hyperglycemia and no-reflow.
Many of the risk factors such as hypertension, diabetes mellitus and female gender that were discussed previously are associated with no-reflow [23]. There was also an association between abnormal vascular function and stroke in this study which was indicated by Kim, et at. [24], although, opposing these results, Mirbolouk, et al. [5] cohort found that there was no significant relationship between no-reflow and female gender and stroke.
This study found that high blood pressure, female gender, acute and chronic kidney injury, high levels of inflammatory biomarkers, involvement of LAD territory, and complex atherosclerotic plaques containing high thrombus burden were of great significance which coincided with Ashoori, et al. [6].
This study also revealed that lower systolic blood pressure was correlated with increased risk of no-reflow independently. It might be related to reduction of coronary arterial perfusion pressure due to decreased blood pressure; this was similar to the results of Mirbolouk, et al. [5]. Moreover, swelled myocardial cells concomitant with interstitial edema might lead to microvascular compression. This mechanism and oxidative stress of ischemic endothelial cells along with vasoconstriction can reduce perfusion of microvasculator and lead to no-reflow [5].
Applying prophylactic intracoronary dilators, primary stenting, short stents with adjusted lower pressure, thrombus aspiration in particular cases, and occasionally distal protection devices may be beneficial [25-27].
In this regard, CHA2DS2-VASc model affords a simple, time saving tool for risk stratification.
Hence, in line with previous studies [28], it was not surprising to observe a considerable association of the score with in-hospital mortality.
This study found greater mortality in STEMI patients older than 70 and among who had heart failure. Previous studies demonstrated established association of age, and reduced EF with MACE of ACS patients. However, it was not observed previously known impacts of gender on mortality or no-reflow [5]. This study had confirmed enhanced mortality and morbidity in the presence of no-reflow phenomenon. Other studies [5,6,16] displayed independent predictive role of diabetes mellitus, which was similar to the results of this study. However, it was found a borderline statistical significance for this relationship. Overall, the question about whether high CHA2DS2-VASc has an independent additional effect beyond its components remains controversial [29].
Previous investigations [5,6,16] detected various determinants of suboptimal reperfusion following primary PCI. Heart failure (embedded in CHA2DS2-VASc score), cardiogenic shock, and high thrombus burden were also found in this study in line with former reports.
This study showed that lower stent diameter & higher stent length can predict no-reflow. Based on these findings grade 0 TIMI flow rate at initial angiography also was an independent predictor of no-reflow similar to another previous study [5].
Bayramoğlu, et al. [14] declared the impacts of long stent (> 20 mm) and thrombus grade on no-reflow risk. Odds ratios 3.607 (1.932–6.734), and 3.139 (1.081–9.113), respectively. This study showed significant association of high thrombus burden with no-reflow incidence.
In consistent with this study, Mirbolouk, et al. [5] demonstrated that initial TIMI flow greater than 1 correlates with lower likelihood of final suboptimal flow (OR: 0.06 (0.02–0.20)).
Significantly higher CHA2DS2-VASc score is associated with higher risk of no- reflow phenomenon and in-hospital mortality rates in patients with STEMI who underwent primary PCI.
Several limitations were observed in this study, the retrospective design based on a single center registry data and the incidence of no-reflow in this study was low.
Acidosis–alkalosis state, blood gas mixture, dosage and types of anticoagulant, protocol of balloon dilation, and pain time to balloon interval are other risk factors that were not considered in this study.
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, et al. ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011; 58: 2550–2583. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22064601
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008; 117: 3152–3156. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18559715
- Durante A, Camici PG. Novel insights into an old phenomenon: the no reflow. Int J Cardiol. 2015; 187: 273–280. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25838230
- Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008; 72: 950–957. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19021281
- Mirbolouk F, Gholipour M, Salari A, Shakiba M, Kheyrkhah J, et al. CHA2DS2-VASc Score Predict No-Reflow Phenomenon in Primary Percutaneous Coronary Intervention in Primary Percutaneous Coronary Intervention. J Cardiovasc Thorac Res. 2018; 10: 46-52. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29707178
- Ashoori A, Pourhosseini H, Ghodsi S, Salarifar M, Nematipour E, et al. CHA2DS2-VASc Score as an Independent Predictor of Suboptimal Reperfusion and Short-Term Mortality after Primary PCI in Patients with Acute ST Segment Elevation Myocardial Infarction. Medicina (Kaunas). 2019; 55: E35. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30717292
- Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: State of the art. Arch Cardiovasc Dis. 2015; 108: 661–674. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26616729
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest. 2010; 137: 263–272. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19762550
- Chan YH, Yiu KH, Lau KK, Lam TH, Lau CP, et al. The CHADS2 and CHA2DS2-VASc scores predict adverse vascular function, ischemic stroke and cardiovascular death in high-risk patients without atrial fibrillation: role of incorporating PR prolongation. Atherosclerosis. 2014; 237: 504–513. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25463082
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, et al. 2013 A CCF/AHA guideline for the management of ST–elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61: e78. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23247304
- Rasoul S, Dambrink JH, Breeman A, Elvan A, van’t Hof AW. The relation between myocardial blush grade and myocardial contrast echocardiography: Which one is a better predictor of myocardial damage? Neth Heart J. 2010; 18: 25–30. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20111640
- Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, et al. A randomized trial of deferred stenting versus immediate stenting to prevent No- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). Am Coll Cardiol. 2014; 63: 2088–2098. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24583294
- Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ. 2006; 333: 1091. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17032691
- Bayramoğlu A, Taşolar H, Kaya A, Tanboğa İH, Yaman M, et al. Prediction of no-reflow and major adverse cardiovascular events with a new scoring system in STEMI patients. J Interv Cardiol. 2018; 31: 144-149. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29193382
- Magro M, Nauta ST, Simsek C, Boersma E, van der Heide E, et al. Usefulness of the SYNTAX Score to Predict “No Reflow” in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 2012; 109: 601–606. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22177003
- Ipek G, Onuk T, Karatas MB, Gungor B, Osken A, et al. CHA2DS2-VASc Score is a Predictor of No-Reflow in Patients with ST-Segment Elevation Myocardial Infarction Who Underwent Primary Percutaneous Intervention. Angiology. 2015; 67: 840–845. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26685178
- Bozbay M, Uyarel H, Cicek G, Oz A, Keskin M, et al. CHA2DS2-VASc Score Predicts In-Hospital and Long-Term Clinical Outcomes in Patients with ST-Segment Elevation Myocardial Infarction Who Were Undergoing Primary Percutaneous Coronary Intervention. Clin Appl Thromb Hemost. 2016; 23: 132–138. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27170782
- Hioki H, Miura T, Miyashita Y, Motoki H, Shimada K, et al. Risk stratification using the CHA2DS2-VASc score in patients with coronary heart disease undergoing percutaneous coronary intervention; sub-analysis of SHINANO registry. IJC Heart Vasc. 2015; 7: 76–81. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28785649
- Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010; 55: 2383–2389. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20488311
- Harrison RW, Aggarwal A, Ou F, Klein LW, Rumsfeld JS, et al. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol. 2012; 111: 178–184. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23111142
- Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N Engl J Med. 2007; 357: 1631–1638. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17942875
- Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol. 2003; 41: 1–7. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12570936
- Dean J, Dela Cruz S, Mehta PK, Merz CNB. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol. 2015; 12: 406–414. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26011377
- Kim J, Cha MJ, Lee DH, Lee HS, Nam CM, et al. The association between cerebral atherosclerosis and arterial stiffness in acute ischemic stroke. Atherosclerosis. 2011; 219: 887–891. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21974846
- Heusch G. Cardioprotection: Chances and challenges of its translation to the clinic. Lancet. 2013; 381: 166–175. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23095318
- Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of No-Reflow Phenomenon in the catheterization laboratory. J Am Coll Cardiolvasc Interv. 2017; 10: 215–223. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28183461
- De Vita M, Burzotta F, Biondi-Zoccai GG, Lefevre T, Dudek D, et al. Individual patient-date meta-analysis comparing clinical outcome in patients with ST-elevation myocardial infarction treated with percutaneous Coronary intervention with or without prior thrombectomy. ATTEMPT study: A pooled Analysis of Trials on Thrombectomy in Acute Myocardial infarction based on individual Patient data. Vasc Health Risk Manag. 2009; 5: 243–247. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19436647
- Chua SK, Lo HM, Chiu CZ, Shyu KG. Use of CHADS2 and CHA2DS2-VASc Scores to Predict Subsequent Myocardial Infarction, Stroke, and Death in Patients with Acute Coronary Syndrome: Data from Taiwan Acute Coronary Syndrome Full Spectrum Registry. PLoS ONE. 2014; 9: e111167. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25343586
- Al-Thani HA, El-Menyar A, Zubaid M, Rashed WA, Ridha M, et al. Peripheral arterial disease in patients presenting with acute coronary syndrome in six middle eastern countries. Int J Vasc Med. 2011; 2011: 815-902. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22220279