More Information
Submitted: 04 November 2019 | Approved: 14 November 2019 | Published: 15 November 2019
How to cite this article: Ajam F, Maludum O, Ugoeke N, Mahida H, Alrefaee A, et al. Left ventricular ejection fraction and contrast induced acute kidney injury in patients undergoing cardiac catheterization: Results of retrospective chart review. J Cardiol Cardiovasc Med. 2019; 4: 195-198.
DOI: 10.29328/journal.jccm.1001066
Copyright License: © 2019 Ajam F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Contrast nephropathy; Left ventricular ejection fraction; Acute kidney injury
Left ventricular ejection fraction and contrast induced acute kidney injury in patients undergoing cardiac catheterization: Results of retrospective chart review
Firas Ajam, Obiora Maludum, Nene Ugoeke, Hetavi Mahida, Anas Alrefaee, Amy Quinlan DNP, Jennifer Heck-Kanellidis NP, Dawn Calderon DO, Mohammad A Hossain* and Arif Asif
Department of Medicine, Jersey Shore University Medical Center, Hackensack Meridian Health, Neptune, NJ 07753, USA
*Address for Correspondence: Mohammad A Hossain, MD, Faculty and Assistant Professor of Medicine, Department of Medicine, Jersey Shore University Medical Center, Core Faculty and Assistant Professor of Medicine, Hackensack-Meridian School of Medicine, Seton Hall, Neptune, USA, Tel: 732-897-3990; ext: 59576; Fax: 732.897.3997; Email: [email protected]
Background: Contrast-induced acute kidney injury (CI-AKI) is an important cause of increasing the hospital stay and in-hospital mortality. By increasing intra-renal vasoconstriction, left ventricular ejection fraction (LVEF) can increase the risk of CI-AKI. We sought to investigate whether LVEF can impact the incidence of CI-AKI after cardiac catheterization and whether it can be used to predict CI-AKI.
Methods: Patients underwent cardiac catheterization from December 2017 to February 2018 at Jersey Shore University Medical Center were enrolled in the study. Contrast-induced acute kidney injury (CI-AKI) was defined as an increase in serum creatinine of ≥ 0.5 mg/dL or an increase of ≥ 25% from the pre-procedure value within 72 hours post-procedure. The maximum allowable contrast dose was calculated using the following formula: (5* (weight (kg)/creatinine level (mg/dL)). A multivariable logistic regression analyses, controlling for potential confounders, were used to test associations between LVEF and CI-AKI.
Results: 9.6% had post catheterization CI-AKI. A total of 18 out of 44 (44%) of patients who had CI-AKI also had ongoing congestive heart failure. No statistically significant association found neither with maximum allowable contrast (p = 0.009) nor ejection fraction (p = 0.099) with the development of CI-AKI.
Conclusion: In spite of the fact that no statistically significant relationship found between the percentage maximum contrast dose and the ejection fraction with the post-procedure CI-AKI, we heighten the essential of employing Maximum Allowable Contrast Dose (MACD) and ejection fraction in patients undergoing PCI to be used as a clinical guide to predict CI-AKI.
Contrast-induced acute kidney injury (CI-AKI) is an important consequential complication following the use of iodinated contrast that ultimately lead to an increase in the length of hospital stay, expenditures and in-hospital mortality [1,2]. Given the rapidly growing number of percutaneous coronary interventions, the number of patients exposed to iodinated contrast has been dramatically increased.
Myocardial ischemia is a major etiology of heart failure and patients undergoing percutaneous coronary interventions have high prevalence of heart failure [3]. Simultaneously, volume expansion with intravenous fluids prior to coronary angiography have been shown to have a protective effect on the kidneys which is a challenging task in patients with concurrent heart failure [3].
Left ventricular ejection fraction (LVEF) is the most frequently used parameter to evaluate cardiac function in patients with heart failure. Because low EF can induce intra-renal vasoconstriction, reduced ejection fraction can serve as an independent risk factor for CI-AKI [4]. Nonetheless, the association between LVEF and contrast induced AKI is still controversial. Therefore, we sought to investigate whether the LVEF can impact the incidence of CI-AKI in patients undergoing cardiac catheterization.
This study is a retrospective analysis of patients who underwent cardiac catheterization from December 2017 to February 2018 at Jersey Shore University Medical Center. We included all adults age > 18 years who underwent either elective or emergent cardiac catheterizations.
Demographic characteristics, comorbidities, baseline renal functions, left ventricular ejection fraction, estimated glomerular filtration rate (eGFR; calculated by CKD–EPI creatinine equation) were recorded. Institutional review board approval was obtained for this study. All study procedures were carried out in accordance with the Declaration of Helsinki regarding research involving human subjects.
For the purposes of this study, contrast-induced acute kidney injury was defined as an increase in serum creatinine of ≥ 0.5 mg/dL or an increase of ≥ 25% from the pre-procedure value within 72 hours post-procedure. The maximum allowable contrast dose was calculated using the following formula: (5* (weight (kg)/creatinine level (mg/dL)) [5].
The percentage of the maximum allowable contrast dose used in each procedure was calculated by dividing the contrast volume used by the estimated maximum allowable contrast dose. The change in creatinine was calculated by subtracting the pre-procedure creatinine level from the maximum creatinine value obtained within 72 hours post-procedure. To maintain directionality of the change, absolute values were not used.
The distribution of continuous variables including age, percentage of the maximum allowable dose of contrast used, and creatinine difference were examined using median, mean, standard-deviation and inter-quartile range, as appropriate. Chi-square test, or Fisher exact test was used, as appropriate, to test associations between categorical variables. The Wilcoxon rank-sum test was used to test non-parametric associations when indicated. Pearson’s correlation was used, as appropriate, to test the strength and direction of linear relationships between continuous variables. Multivariable logistic regression analyses, controlling for potential confounders, were used to test associations. All statistical tests were two-sided, and a p value of ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
A total of 457 patients who underwent cardiac catheterization were included in this study. Of these, 44 patients (9.6%) had post-procedure contrast-induced nephropathy. Baseline characteristics of the patients are shown in table 1. Patients who developed contrast-induced AKI were more likely to be female (p = 0.043), previous myocardial infarction (p = 0.028) and to have had a prior diagnosis of renal impairment (p = 0.049). Only Baseline clinical characteristics between the two groups were otherwise similar. 18 out of 44 (40%) of patients who developed AKI had ongoing CHF. Based on ejection fraction, no statistically significant difference encountered in the incidence of CI-AKI (Table 2).
| Table 1: Demographic Characteristics of the patients (n = 457). | |||
| Contrast-Induced AKI | |||
| Present (n = 44) |
Absent (n = 413) |
p - value | |
| Age (years) (median ± IQR) | 69.03 ± 16.97 | 69.58 ± 18.12 | 0.641 |
| Female | 47.73 (21/44) | 31.96 (132/413) | 0.043 |
| Diabetes mellitus | 40.91 (18/44) | 36.08 (149/413) | 0.527 |
| Hypertension | 90.91 (40/44) | 86.20 (356/413) | 0.382 |
| Dyslipidemia | 81.82 (36/44) | 79.42 (328/413) | 0.707 |
| Previous PCI | 25.0 (11/44) | 36.80 (152/413) | 0.148 |
| PCI | 45.45 (20/44) | 59.81 (247/413) | 0.066 |
| Previous myocardial infarction | 11.4 (5/44) | 26.15 (108/413) | 0.028 |
| Previous CABG | 4.55 (2/44) | 13.32 (55/413) | 0.145 |
| Previous CHF | 25.0 (11/44) | 14.77 (61/413) | 0.084 |
| Current CHF | 40.91 (18/44) | 31.72 (131/413) | 0.238 |
| NYHA Class 1 2 3 4 |
77.27 (34/44) 13.64 (6/44) 2.27 (1/44) 6.82 (3/44) |
85.47(353/413) 5.33 (22/413) 4.84 (20/413) 4.36 (18/413) |
|
| Peripheral vascular disease | 11.36 (5/44) | 13.08 (54/413) | 1.000 |
| Renal impairment | 13.64 (6/44) | 5.57 (23/413) | 0.049 |
| Table 2: Relation of LVEF and CI-AKI. | |||
| Contrast induced- AKI | |||
| LVEF (%) | Present (37) | Absent (322) | p - value |
| ≤ 50% | 51.35 (19/37) | 37.27 (120/322) | 0.099 |
| > 50% | 48.65 (18/37) | 62.73 (202/322) | 1.000 |
Maximum allowable contrast & contrast-induced AKI
A statistically significant, but weakly negative correlation was observed between the percentage of the maximum allowable contrast dose administered and change in creatinine (coefficient of correlation, r = -0.122; p = 0.009). Multivariable logistic regression controlling for age, gender, diabetes mellitus, prior renal insufficiency and prior MI showed no statistically significant relationship between percentage maximum contrast dose and post-procedure AKI (p = 0.352).
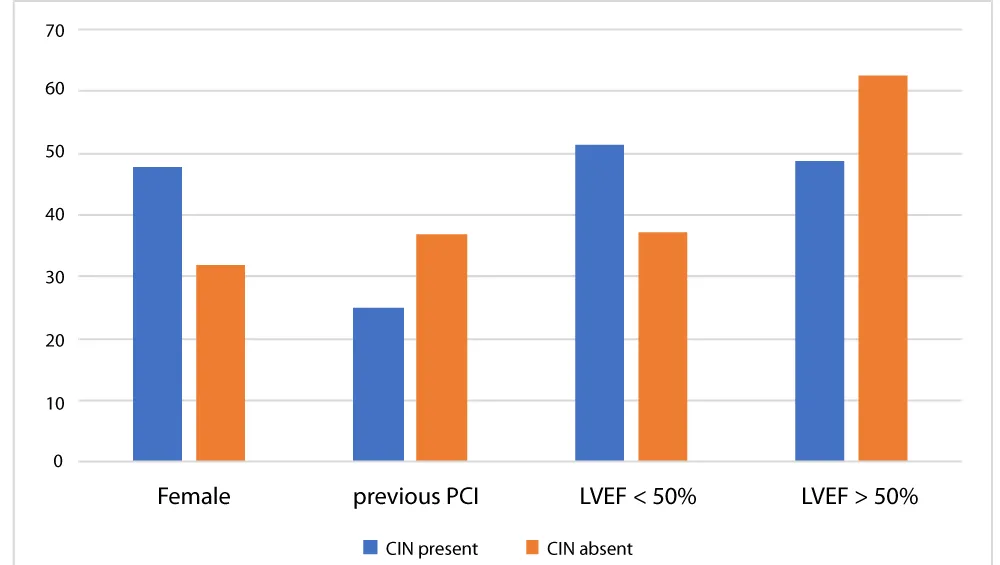
Iodinated contrast can induce several renal hemodynamic changes and alter the renal tubular function. Vasoconstriction of the intrarenal vasculature is the main hemodynamic changes that can lead to renal medullary necrosis [6-10]. Additionally, vasoconstriction can also activate renin-angiotensin system with increase production of reactive oxygen species (ROS) [8]. The production of ROS can alter the regulation of renal tubular transport and magnify the renal hypoxia. Heart failure characterized by low EF is capable of inducing a state of low renal perfusion and over activation of the renin-Angiotensin system, theoretically aggravating the tubular injury from contrast. Our study did not support this hypothesis as no statistically significant difference was found in group with low EF (EF < 50%) when compared with normal EF (EF > 50%) (Figure 1).
Figure 1: Comparison in the percentage of Contrast induced nephropathy between female, previous PCI, LVEF less than 50% and more than 50%.
Despite the controversial association between LVEF and CIN, LVEF is still considered one of the most broadly used measurements to evaluate for cardiac functions associated with hemodynamic instability, and consequently causes inadequate renal perfusion. An observational study done on 386 patients undergoing PCI, patients with declined LVEF had significantly higher rate of CIN compared with those with LVEF 45% (14.4% vs 5.7%; p = 0.02). Additionally, declined LVEF was an independent predictor of CIN [11]. However, studies conducted by Kurtul, et al. and Barbieri, et al. showed an opposite effect after adjusting for several confounders [10]. As observed in all the above-mentioned studies, only a small number of patients with HF were included, and consequently, those studies were unable to analyze the association between LVEF and CIN. Furthermore, HF, as an important risk factor of CIN, was not included in the multivariate analysis. In contrast, our study included sufficient patients with HF and adjusted for the potential confounders to investigate the association of LVEF with CIN following CAG/PCI (Spell out CAG. You are using this for the first time here).
The MACD equation currently used to determine the threshold for safe contrast use, was initially developed by Cigarroa and colleagues [12]. It is calculated by: 5 (mL) of contrast per Kg of body weight / baseline serum creatinine (mg/dL). Cigarroa found that CI-AKI developed in only 2% of patients that had contrast exposure under the calculated threshold, but 38% of patients receiving contrast over the MACD developed CI-AKI [12]. Moreover, other studies have showed similar results, demanding that exceeding the MACD had a significant risk of developing nephropathy [13,14]. In our study, a multivariable logistic regression controlling for common confounders showed no statistically significant relationship between percentage maximum contrast dose and post-procedure AKI likely due to inadequate power and smaller difference between the groups than was expected.
Studies have reported that both elderly and very young patients are particularly disposed to develop CI-AKI [15,16]. Pre-existing kidney disease is a major risk factor for CI- AKI [16]. Additionally, exposure to nephrotoxins is associated with CI-AKI. Other traditional risk factors include: diabetes mellitus, hypertension, cardiovascular disease, chronic liver disease, and chronic obstructive pulmonary disease [18]. Unlike those studies, our study also showed that females are possibly more susceptible to CIN than males.
We acknowledge several limitations of our study. This was a single-center, retrospective, and non-randomized observational study. Because our data was collected from a single medical center, further studies are required from other medical centers to confirm the results. Our study was not accurately able to measure pre-procedure prophylaxis including intravenous fluid or other prophylactic measures. The study captured pre- and post-PCI serum creatinine values. However, the length of hospital stay was not enough to assess the long term effect of iodinated contrast, and some of the patients might not have CI-AKI at the time of the discharge. Despite conducting multivariable analysis and propensity matching to negate any possible confounders, there might be some immeasurable factors.
In spite the fact that we found no statistically significant relationship neither with percentage maximum contrast dose nor ejection fraction with post-procedure AKI, we accentuate the prerequisite of employ the MACD and ejection fraction in patients undergoing PCI to be used as a clinical guide for the procedure. We also recommend adequate hydration, lessens the contrast volume, and discussing the clinical benefits of using iso- and low osmolar contrast. Further studies need to be done regarding the impact of using contrasts with different osmolarity on preventing contrast induced AKI.
- Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC CardiovascInterv. 2014; 7: 1-9. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24456715
- Weisbord SD, Palevsky PM. Contrast-induced acute kidney injury: short and long-term implications. Semin Nephrol. 2011; 31: 300-309. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21784279
- Pakfetrat M, Malekmakan L, Zahra Salmanpour, Mohammad Hossein Nikoo, Peyman Izadpanah, Comparison of Normal Saline, Ringer's Lactate, and Sodium Bicarbonate for Prevention of Contrast-induced Nephropathy in Patients with Coronary Angiography: A Randomized Double-blind Clinical Trial. Indian J Nephrol. 2019; 29: 22-27. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6375023/
- Wang K, Li HL, Bei WJ, Guo XS, Chen SQ, et al. Association of left ventricular ejection fraction with contrast-induced nephropathy and mortality following coronary angiography or intervention in patients with heart failure. Ther Clin Risk Manag. 2017; 13: 887-895. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28769566
- Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, et al. Does safe dosing of iodinated contrast prevent contrast-induced acute kidney injury?. Circ Cardiovasc Interv. 2010; 3: 346-350. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20587788
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002; 39: 930-936. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11979336
- Wong PCY, Li Z, Guo J, Zhang A. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012; 158: 186-192. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21784541
- Caiazza, A, Russo L, Sabbatini, M, Russo D. Hemodynamic and Tubular Changes Induced by Contrast Media. BioMed Research International, 2014; 1-7.
- Hossain MA, Costanzo E, Cosentino J, Patel C, Qaisar H, et al. Contrast-induced nephropathy: Pathophysiology, risk factors, and prevention. Saudi J Kidney Dis Transpl. 2018; 29:1-9. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29456202
- Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004; 44:1393-1399. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15464318
- Flint N, Kaufman N, Gal-Oz A, Margolis G, Topilsky Y, et al. Echocardiographic correlates of left ventricular filling pressures and acute cardio-renal syndrome in ST segment elevation myocardial infarction patients. Clin Res Cardiol. 2017; 106: 20-126. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27550512
- Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989; 86: 649-652. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2729314
- Freeman RV, O'Donnell M, Share D, Meengs WL, Kline-Rogers E, et al. Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. The American journal of cardiology. 2002; 90:1068-1073. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12423705
- Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002; 105: 2259-2264. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12010907
- Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, et al. A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. American Journal of Kidney Diseases. 2015; 66: 591-601. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25943717
- Chang JW, Jeng MJ, Yang LY, Chen TJ, Chiang SC, et al. The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney International. 2015; 87: 632-639. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25252027
- Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. The New England Journal of Medicine. 2014; 371: 58-66. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24988558
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. kdigo clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012; 2: 1-138.