More Information
Submitted: 06 September 2019 | Approved: 19 November 2019 | Published: 20 November 2019
How to cite this article: Rigatto K, Bós DSG, Fernandes R, Jaenisch RB, Lago PD. Only low intensity of aerobic exercise improves respiratory compliance in pulmonary hypertensive rats. J Cardiol Cardiovasc Med. 2019; 4: 205-209.
DOI: 10.29328/journal.jccm.1001069
Copyright License: © 2019 Rigatto K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Phypertension; Monocrotaline; Exercise; Respiratory mechanics; Sympathovagal balance
Abbreviations: PH: Pulmonary Hypertension; ET: Exercise Training; RV: Right Ventricular; RVSP: Right Ventricular Systolic Pressure; RVDP: Right Ventricular End Diastolic Pressure; CO: Control Healthy Sedentary Group; PH-S: Pulmonary Hypertension Sedentary Rats; PH-L: Pulmonary Hypertension - Low Intensity Exercise Training; PH-M: Pulmonary Hypertension - Moderate Intensity Exercise Training; MCT: Monocrotaline; Est: Quasi-static elastance; Cst: Quasi-static compliance
Only low intensity of aerobic exercise improves respiratory compliance in pulmonary hypertensive rats
Katya Rigatto*, Denielli Da SG Bós, Renata Fernandes, Rodrigo B Jaenisch and Pedro Dal Lago
Postgraduate Program in Health Sciences, Federal University of Health Sciences of Porto Alegre, FCSPA. Porto Alegre, Brazil
*Address for Correspondence: Katya Rigatto, Av Sarmento Leite, 245–Prédio 3, Laboratório 503, CEP: 90050-170; Tel: 55 (51) 99998-6655; Email: [email protected]
Objective: To investigate in an animal model of Pulmonary Hypertension (PH) by monocrotaline whether a lower exercise intensity, which has lower potential to provoke dyspnea symptoms, could prevent the increase the right ventricle pressure and the decrease in respiratory compliance.
Setting: A research laboratory. ANIMALS: twenty-one Wistar rats were randomized to the groups: Control (CO; saline solution); PH-sedentary; PH-low and PH-moderate intensity of exercise training (ET).
Interventions: They received a single saline or monocrotaline subcutaneous injection (50 mg/kg). The exercise program was performed during 3-weeks.
Main Outcome Measures: Rats were evaluated by their morphometric and hemodynamic changes and by the respiratory mechanic responses induced by the exercise protocols.
Results: Both protocols of ET significantly (p < 0.05) attenuated the increase in the right ventricular systolic pressure. However, the lower intensity was more effective to prevent the impairment in the respiratory and quasi-static compliance.
Conclusion: Collectively, our results showed for the first time the benefits of ET to the respiratory system mechanics. We also demonstrated that intensity is crucial in PH, probably due to the difficulty to match VO2 capacity and O2 demand during exercise. The improvement in quasi-static compliance not only might improve the ability to breathe, and capture oxygen, but also welfare.
Pulmonary hypertension (PH) is a pathophysiological disorder with high mortality. It causes a progressive increase in pulmonary vascular resistance that induces an increase in right ventricular (RV) pressure [1] and hypertrophy. Despite the advances in the pharmacological treatment [2], patients still suffer from dyspnea, often the most distressing symptom [3].
Studies have been demonstrating the beneficial effects of exercise training (ET) in PH patients[4]. These findings not only changed the conception that exercise is unsafe, but provided evidence that ET could be beneficial also to PH patients.
It is well known that exercise therapy improves quality of life in several conditions. Specific exercise therapy guideline is available for some chronic pulmonary disease [5] but, unfortunately, such robust recommendation is not established for PH. Information about crucial aspects of an exercise intervention, such as frequency and intensity, is mandatory for its safe implementation [6]. Therefore, to study and understand the ET on this disease is essential for developing adequate guidance.
The lungs and chest are formed by tissue with elastic properties. These properties have a fundamental role in the pulmonary ability to expand and retract [7], and consequently, in the ability to provide oxygen for the proper gas exchange in the lungs. Frequently, the lung injury is accompanied by alteration in the lung’s mechanical properties, that can be very specific to the degree and nature of this injury [8]. According to the literature, PH is accompanied by structural changes in pulmonary vasculature [9], which might be associated with an increase in lung resistance and a decrease in its compliance.
Although the mechanism is unknown, ET’s improvement in patients’ functional capacity and quality of life is clear [10,11]. Also, the improvement of different outcomes in rats with PH have been associated with ET [12,13]. On the other hand, the ideal intensity, frequency, timing, duration, or exercise modality for the PH treatment remains unclear. The most common prescription in clinical trials is based on aerobic and strength training in highly supervised programs [12].
In addition, ET is beneficial in stable PH, although the outcome was adverse in progressive PH rats [14]. There are multiple modalities of ET that show different results in humans [11,15,16] but, surprisingly, even though it is crucial to adjust the availability of oxygen to the lungs, there are no reports addressing respiratory mechanics and exercise training in PH. Thus, our goal was to investigate in a PH animal model whether the intensity of ET may change differently the properties of respiratory mechanics.
Ethics requirements
The Ethics Committee approved the protocol (number: 069/11) in accordance with the Guidance for the Description of Animal Research in Scientific Publications [17].
Experimental procedures
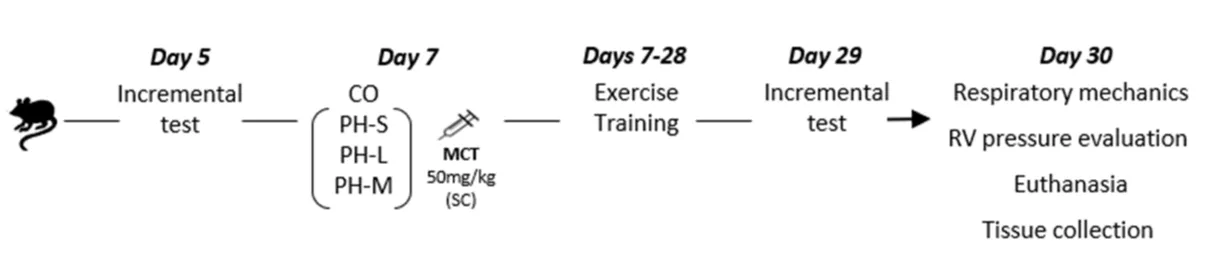
The experiments follow the sequence presented in figure 1.
Figure 1: Experimental sequence. CO: Control Group; PH-S: Sedentary Group; PH-L: Low Intensity Training; PH-M: Moderate Intensity Training; MCT: Monocrotaline; RV: Right Ventricle
Male Wistar rats (70 days old; ~170 g) from the animal facility were housed in a temperature controlled room (22 ± 1°C), 12:12hour light/dark cycle with free access to water and standard food. From day 0 to 7 rats were adapted to the treadmill. To determine the intensity of the ET protocol, in day 5, rats were subjected to the incremental test [18] on a treadmill with electrical stimulation (AVS®). The test started at 0.3 km/h increasing 0.3 km/h every 3 min. The exhaustion was considered the moment at which the rats remained for 15s under electrical stimulation (2 mV). In day 7, they were randomly assigned to four groups: a control healthy sedentary group (CO; n = 4), which received a saline injection; a PH-Sedentary rats group (PH-S; n = 5), a PH-Low intensity ET group (PH-L; average = 10.8 meters/minute; n = 6) and a PH-moderate intensity ET group (PH-M; average = 16.3 meters/minute; n = 6). The PH was induced by a single subcutaneous injection of monocrotaline (MCT; 50 mg/kg; Sigma-Aldrich®) in sterile saline. One sedentary rat died during the experiment, probably due to its sensitivity.
Exercise training program
The exercise program was based on Fenning, et al. protocol [14] and performed during the next 3 weeks (from day 7 to day 28). The trained rats were placed on the treadmill (50 min/d-5 times/week); the PH-M and PH-L groups ran with a constant speed of 60% and 40% of the maximum capacity, respectively, according to the incremental test [19]. The CO and PH-S groups were placed on a stopped treadmill while ET-groups were training. In day 29, rats were evaluated in the treadmill incremental test for the second time.
Respiratory mechanics
Two days after the last exercise session (day 30), rats were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). They were tracheostomized using a rigid-type cannula (2 -mmID) connected to an animal ventilator (Flexi Vent, SCIREQ®, Montreal, PQ, Canada), for mechanical ventilation (90 breaths/min), with a tidal volume of 10 ml/kg using 5 cm H2O positive end-expiratory pressure established by a water column. The constant-phase model was fitted as follows: Zrs=R+jωI+(G-jH)/ωα, where “R”=Newtonian resistance, primarily located in the airways but containing a contribution from the chest wall, “I”=inertance, “G”=coefficient of tissue damping, “H”=co-efficient of tissue elastance, “ω”=angular frequency and “α”=reciprocal frequency-dependent behavior of G & H [20].
Quasi-static elastance (Est) reflects the static elastic recoil pressure of the lungs at a given lung volume, and it was measured by applying the pressure-volume curve technique (the Salazar-Knowles equation), as described by the flexi Vent manufacturer. The respiratory system quasi-static compliance (Cst) reflects the inverse of elastance, measured as the change in volume per unit changed in applied quasi-static pressure [21].
RV pressure evaluation
Immediately after the respiratory mechanics measurements collection, still under ketamine and xylazine anesthesia, the RV pressure was measured using a polyethylene catheter inserted into the right descending jugular vein to reach the RV. To record the RV pressure (sample rate = 2000 Hz/channel), the catheter was connected to a pressure transducer (Strain Gauge Narco Bio-systems Miniature Pulse Transducer RP155, Houston, Texas, USA), coupled to a pressure amplifier (Stemtec®). After tracing stabilization, the pressure was recorded over a 5-min period (CODAS, 1 kHz sampling rate, Dataq® Instruments, Inc., Akron, OH, USA). Data were analyzed on a beat-to-beat basis to determine right ventricular systolic pressure (RVSP) and right ventricular end diastolic pressure (RVDP). Still under anesthesia, rats were killed by decapitation to collect the heart, lungs, and liver for subsequent analyses. The RV and left ventricle were dissected and weighed. The individual ratios of lungs and liver to body weight were calculated to determine the viscera congestion.
Statistical analysis
Data (mean ± SD) were tested for the normal distribution by Shapiro-Wilk test. When confirmed, data were analyzed by one-way ANOVA, followed by the Tukey post hoc test. To detect associations, Pearson or Spearman correlation coefficients were used. The software GraphPad Prism 5 for Windows (GraphPad® Software, San Diego, CA) was used for data analysis. p ≤ 0.05 was considered significant.
Respiratory mechanics
The respiratory system elastance [CO = 1.62 ± 0.43, (n = 4); PH-S = 1.91 ± 0.32, (n = 4); PH-L = 1.48 ± 0.14, (n = 5) and PH-M = 1.75 ± 0.18, (n = 5); F(2.03), p = 0.156] and compliance [CO = 0.68 ± 0.17; PH-S = 0.54 ± 0.09; PH-L = 0.68 ± 0.06 and PH-M = 0.58 ± 0.06; F(2.03), p = 0.121] was similar among groups.
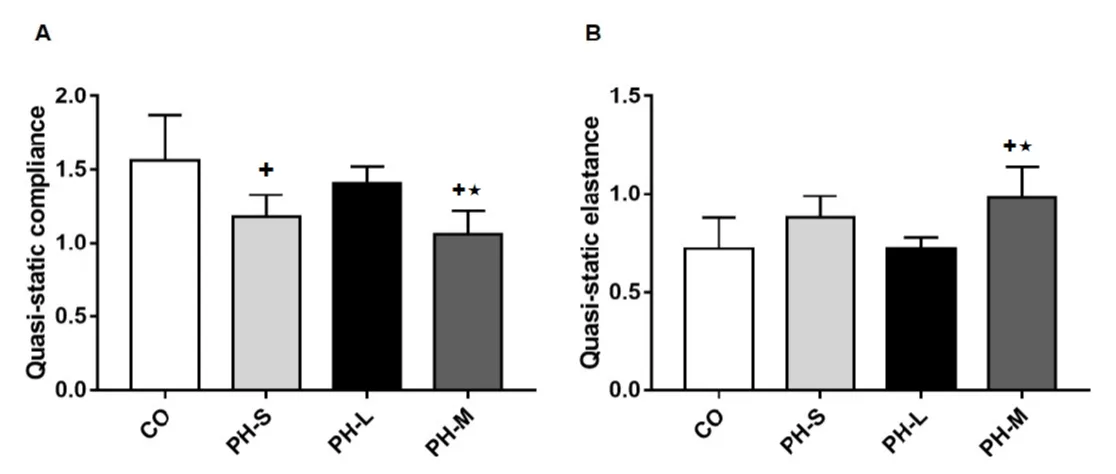
On the other hand, the respiratory system quasi-static elastance [CO = 0.72 ± 0.16; PHS = 0.88 ± 0.11; PH-L = 0.72 ± 0.06 and PH-M = 0.98 ± 0.16; F(4.80), p = 0.01] and quasi-static compliance [ml/cm of water; CO = 1,56 ± 0,31; PH-S = 1,18 ± 0,15; PH-L = 1,41 ± 0,11 and PHM = 1,06 ± 0,16; F(6.40)] were statistically different (p = 0.005). Compared to CO group, the quasi-static compliance was decreased in PH-S and PH-M group; and in PH-M vs PH-L group (p < 0.05). As expected, the quasi-static elastance was increased in the PH-M group versus CO and PH-L (Figure 2).
Figure 2: Panel A: Results of the respiratory system quasi-static compliance; Panel B: The respiratory system quasi-static elastance data. CO: Control Group (n = 4); PH-S: Sedentary Group (n = 4); PH-L: Low Intensity Training (n = 5); PH-M: Moderate Intensity Training (n = 5). Values expressed as mean ± standard deviation; Data were compared by one-way ANOVA followed by Student-Neuman-Keuls test (p < 0.05); +vs CO and ★vs PH-L.
RV hemodynamic
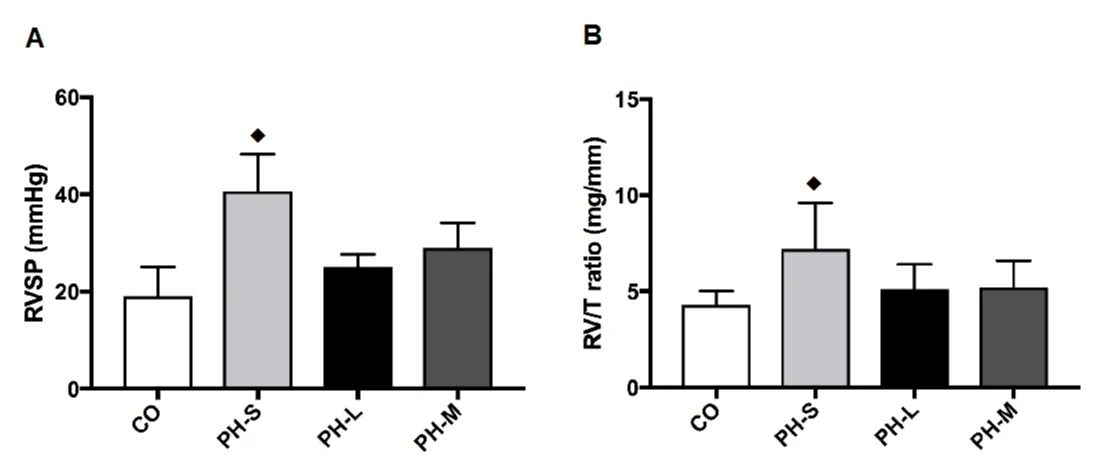
The RVDP was similar among ET groups (Table 1, Figure 3). But, ET significantly prevented the increase in RVSP (PH-L = 37%, and PH-M = 27%) compared with the PH-S group. The heart rate was not significantly different among groups (Table 1).
| Table 1: Right ventricle hemodynamic parameters. | ||||
| Variables | CO (n = 4) | PH-S (n = 5) | PH-L (n = 6) | PH-M (n = 6) |
| RVSP (mmHg) | 19 ± 6.0 | 40 ± 8.6u | 25 ± 2.6 | 29 ± 5.1 |
| RVDP (mmHg) | 3 ± 3.0 | 7.4 ± 1.5 | 3.5 ± 0.2 | 3.9 ± 0.8 |
| HR (bpm) | 290 ± 35 | 283 ± 114 | 321 ± 98 | 315 ± 56 |
| PH: Pulmonary Hypertension; RVSP: Right Ventricular Systolic Pressure; RVEDP: Right Ventricular End Diastolic Pressure; HR: Heart Rate; CO: Control Group; PH-S: Sedentary Group; PH-L: Low Intensity Training; PH-M: Moderate Intensity Training. Values represent means ± standard deviation. up < 0.05 vs. all groups. | ||||
Figure 3: Panel A: RVSP: Results of the right Ventricle Systolic Pressure; Panel B: RV: The ratio between Right Ventricle; weight in mg and the tibia (T) length in mm. CO: Control Group (n = 4); PH-S: Sedentary Group (n = 4); PH-L: Low Intensity Training (n = 5); PH-M: Moderate Intensity Training (n = 5). Values expressed as mean ± standard deviation; Data were compared by one-way ANOVA followed by Student-Neuman-Keuls test ♦(p < 0.05); vs all groups.
Correlations
There were a significant negative and positive correlation between RVSP and Cst (r = -0.587, p = 0.02), and RVSP and Est (r = 0.572, p = 0.03), respectively.
Body and organs data
Before ET, body weight and incremental test performance were similar among groups, but, as expected, the ET increased the speed in the incremental test. Moreover, ET did not change body weight, hepatic congestion and pulmonary congestion [Wet/Dry ratio: CO = 4.01 ± 0.12; PH-S = 5.99 ± 1.52; PH-L = 6.15 ± 1.12 and PH-M = 5.57 ± 0.57; F(0.125), p = 0.943]. On the other hand, significantly (p < 0.05) attenuated right ventricle hypertrophy (Table 2).
| Table 2: Morphometric data, Pulmonary and Hepatic Congestion, and Heart Hypertrophy index. | ||||
| Data/groups | CO (n = 4) | PH-S (n = 5) | PH-L (n = 6) | PH-M (n = 6) |
| BW in the beginning (g) | 182 ± 26 | 145 ± 21 | 128 ± 16 | 138 ± 14 |
| BW in the end (g) | 259 ± 42 | 331 ± 23 | 320 ± 29 | 302 ± 29 |
| IT Before (km/h) | 1.5 ± 0.50 | 1.5 ± 0.21 | 1.4 ± 0.15 | 1.4 ± 0.14 |
| IT After (km/h) | 1.5 ± 0.80 | 1.1 ± 0.39 | 1.8 ± 0.22✚ | 1.8 ± 0.19✚ |
| Lungs/BW (mg/g) | 5.1 ± 1.1 | 9.4 ± 2.9* | 7.4 ± 1.2 | 8.1 ± 1.9 |
| Liver/BW (mg/g) | 32 ± 13 | 41 ± 4 | 41 ± 5 | 40 ± 4 |
| *LV (mg) | 594 ± 121 | 627 ± 62 | 589 ± 79 | 587 ± 80 |
| RV (mg) | 150 ± 24 | 256 ± 83¨ | 183 ± 41 | 185 ± 46 |
| LV/T (mg/mm) | 16.8 ± 3.3 | 17.4 ± 2.4 | 16.4 ± 1.8 | 16.4 ± 1.8 |
| RV/T (mg/mm) | 4.3 ± 0.7 | 7.2 ± 2.4¨ | 5.1 ± 1.3 | 5.2 ± 1.4 |
| BW: Body Weight; PH: Pulmonary Hypertension; IT: Incremental Test; Lungs/BW: Lungs Weight/BW Ratio; Liver/BW: Liver Weight/BW Ratio; LV/T: Left Ventricle Weight/Tibia Length Ratio, *Weighed With The Septum; RV/T: Right Ventricle/Tibia Length Ratio. CO: Control Group; PH-S: Sedentary Group; PH-L: Low Intensity Training; PH-M: Moderate Intensity Training. The “n” Represents the rats from both sets and also, the ones that died due to anesthesia. Values represent means ± standard deviation. *p < 0.05 vs CO; ✚p < 0.03 vs PH-S; ¨p < 0.05 vs All Groups | ||||
The main result noticed on this study is the difference between the two ET groups: although both were well tolerated, only the lower intensity prevented a significant decrease in respiratory and quasi static compliance. This indicates a importance in the intensity of ET in PH to attenuate the impairment of respiratory mechanics. PH is associated with progressive destruction of the pulmonary vascular bed and increased right ventricular pressure and failure. It has been suggested that ET modulates the vasculature and may induce different responses according to the stage of PH [14]. In addition, PH leads to an impairment of exercise capacity [3,13], which should be considered for establishing an ET protocol.
In fact, Handoko, et al. [14] demonstrated in rats treated with MCT that the ET was beneficial in stable, but detrimental in progressive PH. This study showed an increase in wet lung weights in the progressive phenotype without effect in the stable PH. According to these authors, the severity of PH induces different responses to an exercise protocol. Moreover, similarly to our results, no differences were found among PH groups in the lungs wet/dry mass ratio, suggesting that the pulmonary vascular remodeling is modulated by exercise, not by the edema.
ET also attenuated RVSP and RV hypertrophy, as indicated by the RV/T ratio. This attenuation is crucial to reduce the pressure and, thus, the risk of death associated with PH [22]. This could be due to the improvement in arterial vessel morphology. The dyspnea and the fatigue associated with ischemia, inflammation, pulmonary edema and progressive RV failure found in dogs [23] were also probably present in our study seen by the negative correlation between RVSP and Cst, confirmed by a positive correlation with Est. These results were close to that found by Reis, et al. [24] that also showed, in a model of acute lung injury, the decrease in pulmonary resistance and elastance associated with low-intensity of ET. We believe the exercise intensity, in PH, is probably crucial for the respiratory mechanics to match O2 uptake to the O2 demand. The respiratory system difficulties to perform gas exchanges could impair the exercise performance and should be considered to decide the best ET protocol in PH.
Our protocol is the first to compare the effect of two intensities of ET on respiratory mechanics in a MCT model of PH. The quasi-static compliance was lower and the quasistatic elastance was higher in PH-M group, compared to CO and PH-L groups. This result indicates low-intensity ET is more suitable to the respiratory system because it permits to adjust the gas exchange to the metabolism with more efficiency.
In addition, importantly, compared with the PH-S group, the PH-L also presented higher quasi-static compliance and re-established the elastance, improving the respiratory system mechanics in PH. These findings agree with Reis, et al. [24], who demonstrated, in a model of acute lung injury, that low-intensity aerobic ET decreases pulmonary resistance. This effect was not observed in the PH-M group, drawing the attention to the importance of recommending an individualized ET protocol. Therefore, we believe the intensity of ET is crucial to match oxygen blood availability with tissue oxygen uptake during exercise, especially when the pulmonary system is compromised.
Considering that the degree of respiratory mechanics compromise might reflect the severity of pulmonary injury [25] and that the lower exercise intensity attenuated the decrease in complacency, our results do confirm the benefits of ET also over the respiratory mechanics and emphasize the importance of establishing a more effective personalized exercise protocol in PH. On the other hand, we did not explore the mechanism by which the exercise improved the respiratory mechanics. Tshis represents a limitation of our study. However, our first goal is to contribute for improving patient’s functional capacity and quality of life using simple methods, available for everyone. In the future, whether these data are confirmed in PH patients, the reduction in ET intensity might increase the adhesion and the benefits of treatment.
Research supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant 400307/2014-6).
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. J Am Coll Cardiol. 2009; 53: 1573–1619. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19389575
- Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ (Online). 2018. 360: j5492. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29540357
- Dumitrescu D, Sitbon O, Weatherald J, Howard LS. Exertional dyspnoea in pulmonary arterial hypertension. Eur Respir Rev. 2017. 26: 170039. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28877974
- Buys R, Avila A, Cornelissen VA. Exercise training improves physical fitness in patients with pulmonary arterial hypertension: A systematic review and meta-analysis of controlled trials. BMC Pulm Med. 2015. 15: 40. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25896259
- Bolton CE, Blakey JD, Morgan MD. The British Thoracic Society guideline on pulmonary rehabilitation in adults: Your opinion is noted. Thorax. 2014; 69: 388-389. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24430178
- McGregor G, Powell R, Finnegan S, Nichols S, Underwood M. Exercise rehabilitation programmes for pulmonary hypertension: A systematic review of intervention components and reporting quality. BMJ Open Sport and Exercise Medicine. 2018; 4: e000400. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30364456
- Brooks G. Respiratory Physiology-The Essentials. Cardiopulm. Phys Ther J. 2018.
- Allen G, Bates JH. Dynamic mechanical consequences of deep inflation in mice depend on type and degree of lung injury. J Appl Physiol. 2004; 96: 293–300. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12949024
- Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, et al. Evaluation of Pulmonary Artery Stiffness in Pulmonary Hypertension With Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2009; 2: 286-295. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19356573
- Mereles D, Ehlken N, Kreuscher S, Ghofrani S, Hoeper MM, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006; 114: 1482-1489. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16982941
- Grünig E, Ehlken N, Ghofrani A, Staehler G, Meyer FJ, et al. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration. 2011; 81: 394–401. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21311162
- Brown MB, Neves E, Long G, Graber J, Gladish B, et al. High-intensity interval training, but not continuous training, reverses right ventricular hypertrophy and dysfunction in a rat model of pulmonary hypertension. Am J Physiol. Regul Integr Comp Physiol. 2017; 312: R197–R210. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27784688
- Enache I, Favret F, Doutreleau S, Goette Di Marco P, Charles AL, et al. Downhill exercise training in monocrotaline-injected rats: Effects on echocardiographic and haemodynamic variables and survival. Arch Cardiovasc Dis. 2017; 110: 106–115. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28117249
- Handoko ML, de Man FS, Happé CM, Schalij I, Musters RJ, et al. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation. 2009; 120: 42–49. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19546388
- Ehlken N, Lichtblau M, Klose H, Weidenhammer J, Fischer C, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: A prospective, randomized, controlled trial. Eur Heart J. 2016; 37: 35-44. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26231884
- Fox BD, Kassirer M, Weiss I, Raviv Y, Peled N, et al. Ambulatory rehabilitation improves exercise capacity in patients with pulmonary hypertension. J Card Fail. 2011; 17: 196-200. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21362526
- National Research Council. NRC and National Research Council. Guidance for the Description of Animal Research in Scientific Publications. 2011. PubMed: https://www.ncbi.nlm.nih.gov/books/NBK84205/
- Pilis W, Zarzeczny R, Langfort J, Kaciuba-Uściłko H, Nazar K, et al. Anaerobic threshold in rats. Comp Biochem Physiol Comp Physiol. 1993; 106: 285–289. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7902799
- Shepherd RE, Gollnick PD. Oxygen uptake of rats at different work intensities. Pflügers Arch. 1976; 362: 219–222. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/944429
- Jaenisch RB, Hentschke VS, Quagliotto E, Cavinato PR, Schmeing LA, et al. Respiratory muscle training improves hemodynamics, autonomic function, baroreceptor sensitivity, and respiratory mechanics in rats with heart failure. J Appl Physiol. 2011; 111: 1664–1670. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21903877
- Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, et al. Evaluation of Pulmonary Artery Stiffness in Pulmonary Hypertension With Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2009; 2: 286-295. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19356573
- Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009; 34: 1219–1263. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19749199
- Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004; 97: 1119–1128. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15333630
- Reis Gonçalves CT, Reis Gonçalves CG, de Almeida FM, Lopes FD, dos Santos Durão AC, et al. Protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit Care. 2012; 16: R199. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23078757
- Allen G, Bates JHT. Dynamic mechanical consequences of deep inflation in mice depend on type and degree of lung injury. J Appl Physiol. 2004; 96: 293-300. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12949024