More Information
Submitted: 25 October 2019 | Approved: 22 November 2019 | Published: 25 November 2019
How to cite this article: Dang D, Trevisan L, Bouet J, Taieb J. Resolved complete atrioventricular block and left ventricular severe dysfunction in patient with Wegener’s granulomatis after cyclophosphamide and corticosteroid treatment. J Cardiol Cardiovasc Med. 2019; 4: 216-218.
DOI: 10.29328/journal.jccm.1001071
Copyright License: © 2019 Dang D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Resolved complete atrioventricular block and left ventricular severe dysfunction in patient with Wegener’s granulomatis after cyclophosphamide and corticosteroid treatment
Duc Dang*, Lory Trevisan, Jérôme Bouet and Jérôme Taieb
CH of the Pays d’Aix, Cardiology and Vascular Diseases Department, Aix-En-Provence, F-13100, France
*Address for Correspondence: Duc Dang, Cardiology and Vascular Diseases Department, Aix-En-Provence, F-13100, France, Tel: +33 442 339 028; Email: [email protected]
Wegener’s granulomatosis is a systemic granulomatous focus on small to medium sized vessels. It typically affects sinuses, lungs and kidneys due to necrotizing granulomatous vasculitis. Less commonly, cardiac involvement is reported up to 8%-44% of cases [1-3]. It often rises to supraventricular arrhythmia, left ventricular systolic dysfunction, pericarditis, myocarditis, and valvulitis [4,5].
Cardiac conducting tissue involvement is rare and associated with increased mortality. It was only reported in fourteen previous cases, some of them were reversible to medical treatment [6].
A 59-year-old man was admitted to a local hospital for deterioration of the general status associated with dyspnea and an important loss of weight since two weeks.
The total computed tomography body scan was managed without any disorder. BNP was elevated at 3290 µmol/l (< 200 µmol/l). The cardiac checkup revealed a sinus tachycardia (heart rate 100 per minute) and a severed dilated phenotype with a 30% left ventricular ejection fraction (LVEF) measured at transthoracic echography. A treatment with ß-blocker channel (bisoprolol 2.5 mg per day), ACE inhibitor and ceiling loop diuretic was introduced.
Later, the patient came back to the emergency department for worse asthenia and anorexia symptoms. Clinical exam showed a correct blood pressure and some purpura on his legs. A severe renal insufficiency was found with 926 µmol/l of creatinine serum but potassium serum level was normal at 5.1 mmol/l (3.5-5.1 mmol/L). The electrocardiogram (ECG) didn’t find any sign of hyperkalemia. Dialysis was not indicated, as diuresis was not affected.
ACE inhibitor, ceiling loop diuretic and ß-blocker channel were interrupted one week after their introduction.
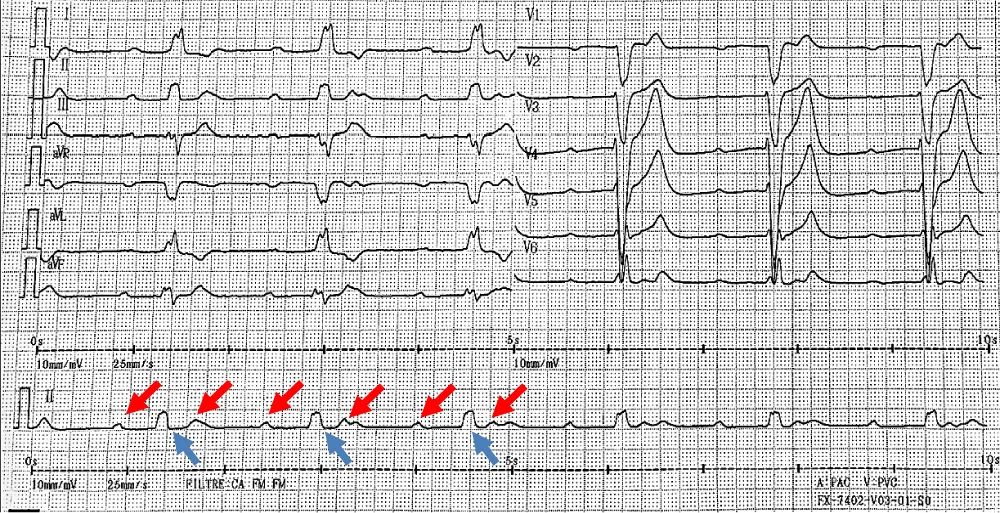
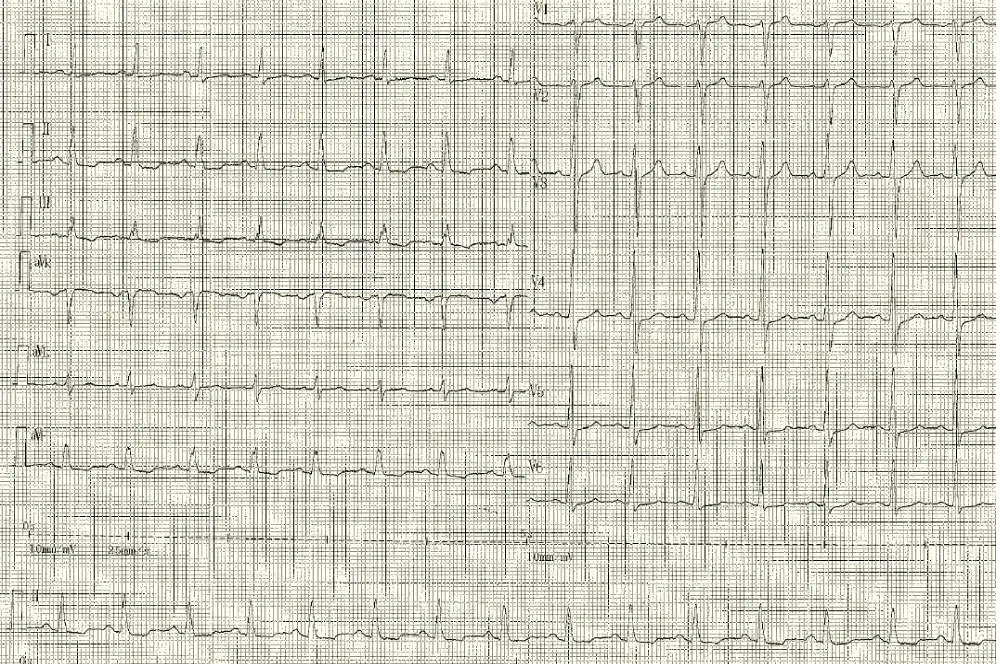
The next day, the patient presented hypotension and bradycardia. The ECG revealed a severe bradycardia (Figure 1, section A) with a complete atrio ventricular (AV) block and wide QRS complex. The blood sample was unchanged. An external pacemaker was implanted by right jugular access and the patient was transferred in a resuscitation care unit to receive ciclophoasphamide and corticosteroid therapy associated to plasma exchange. Few days after immunotherapy, surface ECG showed a sinus tachycardia with normal atrioventricular conduction and narrow QRS complex (Figure 1, Section B).
Figure 1: Section A: Third degree atrioventricular block with complet dissociation between P waves (red arrows) and QRS complexes (blue arrows).
Figure 1: Section B: Sinus rhythm with normal atrioventricular conduction four days after immunotherapy.
Moreover, a week later, there was an improvement of renal function with 200 µmol/l of creatinine serum. At the same time, LVEF increased to 55% at transthoracic echography. The pacemaker interrogation showed 100% of spontaneous ventricular detection. Though, the external pacemaker was removed.
The diagnosis of Wegener’s granulomatosis was established with positive antineutrophil cytoplasmic antibodies against proteinase antigen (ANCA-PR3) and some granulomatosis found on vocal chords during nasofibroscopy.
A cardiac Magnetic Resonance Imaging (MRI) performed one month after treatment initiation found normal right and left ventricular systolic function. LVEF was measured 61% with MRI versus 55% at transthoracic echography 3 weeks before.
There was neither myocardial late enhancement nor cardiac granulomatosis.
After a 3 months of follow up, the patient was asymptomatic. The transthoracic echographia was strictly normal and Holter ECG at one and two months after discharge didn’t find any atrioventricular conduction disturbance.
A permanent pacemaker had never been implanted in this case of AV block in a vasculitis context. The cardiac MRI excluded the presence of cardiac granulomatosis or fibrosis. Many cases of reversible AV block have been described after immunotherapy treatment [6,7]. The AV block and LVEF dysfunction may be related to an inflammation of myocardium and nodo hissian conduction pathway. It was completely reversible after an early immunotherapy treatment in our case report. We suppose that early treatment could stop progression to chronic form of the disease.
However, when cardiac involvement is more severe, cardiac inflammatory may lead to granulomatous inflammatory, microabcesses and collagen necrosis. It could then create chronic conduction disorders. associated with valvulitisis [8,9]. Cassidy, et al. [6] described a patient with a Wegener syndrom in remission since 2 years who presented a permanent complete AV block. For this reason, strict cardiovascular follow up should be performed.
When complete AV block appears in a context of vasculitis suspicion, temporary external pacemaker should be used. An early immunotherapy treatment may lead to complete recovery of atrioventricular conduction and ventricular systolic function.
- Grant SC, Levy RD, Venning MC, Ward C, Brooks NH. Wegener’s granulomatosis and the heart. Br Heart J. 1994;71: 82‑86. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC483617/
- Goodfield NE, Bhandari S, Plant WD, Morley-Davies A, Sutherland GR. Cardiac involvement in Wegener’s granulomatosis. Br Heart J. 1995; 73: 110‑115. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7696016
- Pinching AJ, Lockwood CM, Pussell BA, Rees AJ, Sweny P, Evans DJ, et al. Wegener’s granulomatosis: observations on 18 patients with severe renal disease. Q J Med. 1983; 52: 435‑460. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6657912
- Forstot JZ, Overlie PA, Neufeld GK, Harmon CE, Forstot SL. Cardiac complications of Wegener granulomatosis: a case report of complete heart block and review of the literature. Semin Arthritis Rheum. 1980; 10: 148‑154. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7292019
- Oliveira GHM, Seward JB, Tsang TSM, Specks U. Echocardiographic findings in patients with Wegener granulomatosis. Mayo Clin Proc. 2005; 80: 1435‑1440. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16295023
- Cassidy CJ, Sowden E, Brockbank J, Teh LS, Ho E. A patient with Wegener’s granulomatosis in apparent remission presenting with complete atrioventricular block. J Cardiol Cases. 2011; 3: e71‑74. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30532841
- Wilcke JTR, Nielsen PK, Jacobsen TN. Reversible complete heart block due to Wegener’s granulomatosis. Int J Cardiol. 2003; 89: 297‑298. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12767556
- Ruisi M, Ruisi P, Finkielstein D. Cardiac manifestations of Wegener’s granulomatosis: Case report and review of the literature. J Cardiol Cases. 2010; 2: e99‑102. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30524597
- Strizhakov LA, Krivosheev OG, Kogan EA, Fedorov DN, Semenkova EN, et al. [Aortal regurgitation and atrioventricular block III in Wegener’s granulomatosis]. Klin Med (Mosk). 2007; 85: 68‑71. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18318172