More Information
Submitted: 11 November 2019 | Approved: 25 November 2019 | Published: 26 November 2019
How to cite this article: Bruening T, Al-Khaled M. Readjustment of antithrombotic therapy in stroke-patients owing to transesophageal echocardiography findings. J Cardiol Cardiovasc Med. 2019; 4: 219-226.
DOI: 10.29328/journal.jccm.1001072
Copyright License: © 2019 Bruening T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Readjustment of antithrombotic therapy in stroke-patients owing to transesophageal echocardiography findings
Toralf Bruening* and Mohamed Al-Khaled
Department of Neurology, Ratzeburger Allee 160, 23538 Lübeck, Germany
*Address for Correspondence: Toralf Bruening, Department of Neurology, Ratzeburger Allee 160, 23538 Lübeck, Germany, Tel: +49-(0) 151/67104832; Email: [email protected]
Objectives: Cardioembolic etiology is a frequent source of ischemic stroke. Echocardiogram is the mainstay of cardioembolic source detection with regard to plan secondary stroke management, however it remains unclear how often clinically actionable findings are provided hereby. In addition, it is uncertain whether echocardiography should be performed transthoracic or transesophageal (TEE). In a monocenter study, we evaluated the frequency of pathological findings from TEE evaluation in patients with ischemic stroke with suspected cardioembolic and cryptogenic source and determined whether there was an associated adjustment in the prescribed administration of antithrombotic therapy.
Materials and Methods: Over a 21-month period (2012-2013), we enrolled 143 patients in a prospective monocenter study (mean age ± standard deviation, 70 ± 12 years; females, 44.1%) who were admitted to the Department of Neurology at the University of Lübeck due to ischemic stroke and who underwent TEE due to supposed cardiac embolism. We assessed the presence of atrial fibrillation; days from admission to TEE; and TEE findings, including atrial septal aneurysm, thrombogenic aortic arch, valve failure, presence of left atrial thrombus, and patent foramen ovale. Demografic information and medical history were drawn from patient records and the hospital information system.
Results: On average, TEE was performed 4 days after admission to the hospital. Left atrial thrombus was detected in 3 patients (2.1%), patent foramen ovale (PFO) in 27 (18.9%), atrial septum aneurysm in 17 (11.9%), and thrombogenic aortic arch in 29 (20.3%). Findings from TEE were commonly associated with therapeutic adjustment; antiplatelet therapy increased from 30.1% to 80.4%, oral anticoagulation therapy increased from 2.8% to 27.3%.
Conclusion: Findings from TEE for the evaluation of ischemic stroke lead to frequent adjustment of prior antithrombotic therapy, antiplatelet as well as anticoagulation.
In high-income countries stroke is the third leading cause of death and might account for relevant morbidity in those who survive [1]. Ischemic stroke is primarily due to thrombotic or embolic events, and the heart frequently is the place of origin of emboli [2].
Cardioembolic etiology is the second highest cause of ischemic stroke, accounting for 25% to 30% of all strokes. Cardioembolic stroke is more disabling than stroke with a nonembolic origin, due to the occlusion of larger intracranial arteries and ischemic brain volume [3-6]. Abrupt onset of maximal deficit, epileptic seizures, and accompanying hemorrhagic transformation are the clinical features most commonly associated with cardioembolic stroke [7]. Atrial fibrillation associated with increasing age is the most frequent source of cardiac embolism, especially due to thrombus from the left atrial appendage, and these generally require anticoagulation. Other cardioembolic sources include severe cardiac insufficiency, impaired systolic function and wall-motion abnormalities in cases of cardiomyopathy or owing to myocardial infarction, thrombus in the left atrium or ventricle, valve failure, infective endocarditis or rare findings like atrial myxoma [8].
According to European Stroke Organisation (ESO) guidelines beside a good medical history, physical examination, laboratory testing and 24h 12 lead electrocardiogram (ECG) transthoracic echocardiogram (TTE) is the mainstay of cardioembolic source detection with a grade A recommendation [9]. In patients with embolic stroke of undetermined etiology despite recommended diagnostic work up, who would be eligible for PFO closure screening with bubble test-transcranial Doppler or transesophageal echocardiogram (TEE) is recommended. Beyond that TEE is still the gold standard for aortic arch atheroma (AAA) evaluation, even though screening of AAA with CTA (computed tomography aniography) or TTE is recommended in embolic strokes of undetermined source (ESUS) [9].
In contrast to the recommendation to perform echo-cardiography with regard to plan secondary stroke management, it remains unclear how often clinically actionable findings are provided hereby [10,11]. In a systematic review and meta-analysis Katsanos, et al. [12] found the prevalence of cardiac conditions considered to be causally associated with cerebral ischemia to be low.
The value of routine echocardiography in the management of stroke has been investigated in previous studies that revealed a range of conflicting estimates because of changing opinions on what pathology is considered clinically relevant [13,14]. Where dilated cardiomyopathy no longer requires anticoagulation PFO is now clinically actionable among patients with cryptogenic stroke [15-17].
However, neither current neurological stroke clinical practice nor cardiological guidelines reflect these nuanced data, making it challenging for physicians to decide in which patients echocardiography after an ischemic stroke should be performed, and furthermore which method transthoracic (TTE) or transesophageal (TEE) echocardiography should generally be chosen [10,18]. Especially in elderly patients (older than 65 years) with suspected embolic neurological events routine TEE appears to be unwarranted [19]. Both methods have advantages and disadvantages. Because it is a noninvasive bedside examination, TTE is easy to carry out at any time and without special preparation. However, informative diagnostic value can be limited due to ultrasound conditions (eg, overweight patient). The transesophageal echocardiography provides a better representation of certain parts of the heart and the thoracic aorta due to the close positional relationship between the esophagus and the heart. In addition, small thrombi, especially in the atrial appendage of the left atrium, are better detected. Even in very obese patients, this form of echocardiography may be necessary if a transthoracic echo does not provide satisfactory imaging. On the other hand, TEE generally requires patients to be sedated, with appropriate preparation and surveillance. A study by de Bruijn, et al. [20] proved TEE superior to TTE for identification of cardiac embolic sources in patients with TIA and stroke and furthermore in patients with normal TTE, a cardiac source of embolism was detected by TEE in 39% of patients. Further studies of the diagnostic benefits of TTE and TEE in stroke patients have had mixed results. In all studies, the number of patients with clear therapeutic consequences solely resulting from TEE findings has been small [21,22], including less than 1% of 1.833 stroke patients with sinus rhythm [23]. After a systematic review and meta-analysis of cohort studies of consecutive patients with “cryptogenic” ischemic stroke it remains unclear if routine use of TEE in patients with cryptogenic ischemic stroke is indicated [24]. Haeusler, et al. [25] demonstrated that the diagnostic information of cardiovascular magnetic resonance imaging seems to be complementary to TEE but is not replacing it after acute ischemic stroke. An abnormality on TEE was common (71%) in a study by Galougahi, et al. [26]. On the other hand echocardiography altered management in only 3-5% of subjects referred for stroke assessment [26,27]. Although TTE is unlikely to cause direct patient harm it do may cause indirect harm, e.g., where incidental findings lead to invasive procedure, e.g. TEE, and hence expose patient to further risks.
The objective of our monocenter study was to evaluate the quantity of clinically actionable findings and hence the value of TEE for secondary stroke prevention in patients with cardioembolic and cryptogenic ischemic stroke, and its impact on the actual change of antithrombotic treatment.
Study design
From all patients admitted to the Department of Neurology at the University of Lübeck with ischemic stroke over a 21 month period (2012-2013) we enrolled all patients in our monocenter study who underwent TEE due to supposed cardioembolic or cryptogenic etiology. Stroke patients were treated by neurologists, and the stroke diagnosis was made by at least one vascular neurologist after completing an evaluation of stroke and during the hospital stay. According to national and international guidelines, patients with stroke are generally hospitalized and treated in a stroke unit so as to receive rapid evaluation of the cause of the stroke.
Data source
Demographic information and baseline characteristics, including age, gender, stroke symptoms, medical history, diagnostic and therapeutic procedures, and secondary prevention strategies were drawn from patient records and the hospital information system (Table 1). Patients who were admitted with transient ischemic attack (TIA), stroke mimics, such as epileptic seizures, migraines, or who suffered another functional disorder after diagnostic procedures were completed and during hospitalization were not included in the study.
| Table 1: Patient characteristics. | |
| Characteristics | N = 143 |
| Age, mean (SD) Age above 65, n (%) |
70 (11.6%) 100 (69.9%) |
| Females, n (%) | 63 (44.1%) |
| Medical history, n (%) | |
| Hypertension | 114 (79.7%) |
| Diabetes mellitus | 43 (30.1%) |
| Hyperlipidemia | 56 (39.2%) |
| Previous stroke | 37 (25.9%) |
| Previous myocardial infarction | 21 (14.7%) |
| Prior atrial fibrillation | 8 (5.6%) |
| Premedication, n (%) | |
| Antiplatelet drugs (ASS, P2Y12 inhibitor Clopidogrel, PD-5-inhibitor Dipyridamol+ASS) | 43 (30.1%) |
| Oral anticoagulation (Phenprocoumon, Rivaroxaban, Dabigatran, Apixaban) | 4 (2.8%) |
| Statins | 31 (21.7%) |
| IV rt-PA | 12 (8.4%) |
| Endovascular procedure (thromboendarterectomy) | 4 (2.8%) |
| Neuroimaging procedures, n (%) | |
| cCT | |
| Early signs of ischemia | 52 (36.4%) |
| Follow up CT scan performed | 45 (31.5%) |
| Infarct demarcation in follow-up CT scan | 35 (24.5%) |
| cMRI | 76 (53.1%) |
| Diffusion impairment in MRI | 69 (48.3%) |
| Infarction territory, n (%) | |
| Middle cerebral artery | 83 (58%) |
| Posterior cerebral artery | 15 (10.5%) |
| Anterior cerebral artery | 7 (4.9%) |
| Brain stem | 20 (14%) |
| Other | 28 (19.6%) |
| Ultrasound of brain and neck arteries | 139 (97.2%) |
| Pathological findings in ultrasound | 57 (39.9%) |
| Cardiovascular work-up, n (%) | |
| Transthoracic echocardiogram | 101 (70.6%) |
| Holter ECG | 131 (91.6%) |
| Atrial fibrillation found in holter ECG | 5 (3.5%) |
| 24-hour holter ECG | 120 (83.9%) |
| Atrial fibrillation in 24-hour holter ECG | 6 (4.2%) |
| ASS: Acetylsalicylic Acid; IV RT-PA: Intravenous Recombinant Tissue Plasminogen Activator; CCT: Cranial Computed Tomography; CMRI: Cranial Magnetic Resonance Imaging; ECG: Electrocardiography | |
Study population
We identified patients with a most responsible discharge diagnosis of ischemic stroke using the International Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) diagnosis criteria codes for ischemic stroke. We did not exclude patients who received tissue plasminogen activator (rtPA) and endovascular treatment from our study.
Study outcomes
The primary outcome was the proportion of patients with an echocardiogram with clinically actionable findings for secondary stroke prevention. These were defined using the ESO and American Heart Association (AHA) stroke guidelines. Clinically actionable findings for secondary stroke prevention included patent foramen ovale, atrial and ventricular thrombus, atrial myxoma and valvular vegetation. As a secondary outcome, we quantified the proportion of transition of subjects from antithrombotic therapy before TEE to the state after TEE.
TEE
The TEE investigation was performed as part of the stroke evaluation at the Department of Cardiology by cardiologists who were not involved in the study. Echocardiogram results were extracted from final reports produced by a cardiologist trained in echocardiography. Indications to perform TEE were made by the attending vascular neurologists due to supposed cardiac embolism (e.g. territorial infarct on diagnostic imaging, unproven atrial fibrillation).
Standard protocol approval, registration, and patient consent
This study was part of a benchmarking project (Quality of Treatment of Stroke in the Federal State Schleswig Holstein). The study was approved by the local ethics committee at the University of Lübeck.
Statistical analysis
Data were analyzed using SPSS (version 23; IBM SPSS Statistics, Armonk, NY). Descriptive statistics were calculated, including means and standard deviations (SD) for continuous variables, medians and interquartile ranges (IQR) for scores, and absolute numbers and percentages for nominal and categorical variables.
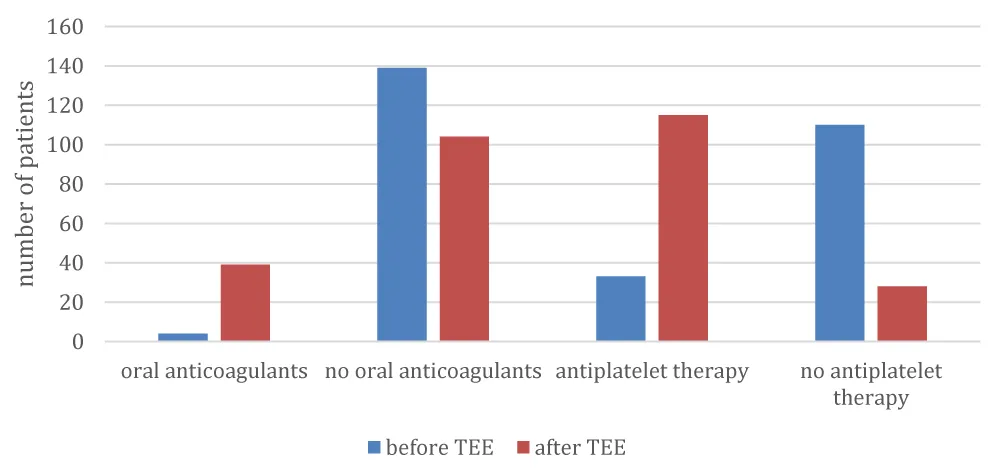
During a 21-month period from January 2012 to September 2013 a total of 143 stroke patients (mean age ± SD, 70 ± 12; females, 44%) met the study inclusion criteria; specifically, TEE was carried out on patients who presented with ischemic stroke, possibly due to cardioembolic or cryptogenic source. Of all patients, 101 (70.6%) had transthoracic echocardiogram in addition to TEE. The median time between a patient’s admission to hospital and the TEE investigation was 4 days (IQR, 0-7). Prior atrial fibrillation was known in 8 patients (5.6%), and was found in 5 additional patients in casual electrocardiography (ECG) and in 6 patients in 24-hour holter ECG. Overall, 62 patients (43%) had a normal echocardiogram. The most frequent TEE findings (Table 2) were thrombogenic aortic arch (20.3%), patent foramen ovale (PFO; 18.9%), and atrial and septum aneurysm (11.9%). Left atrial thrombus (LAT) was found in 3 patients (2.1%), and other thrombi in 6 patients (4.2%). Forty-three patients (30.1%) were taking antiplatelets at the time of admission. By the time of discharge (Table 3), the number of patients taking antiplatelets had increased to 115 (80.4%), most frequently based on pathological findings in ultrasound of brain and neck vessels. The number of patients taking oral anticoagulants increased from 4 (2.8%) to 39 (27.3%), most likely based on findings in TEE. Transition of antithrombotic therapy is shown in figure 1. The quantity of patients treated with statins increased from 31 (21.7%) to 110 (76.9%). Six patients (4.2%) were affected by a recurrence of stroke during hospitalization. All 143 patients survived the hospital stay.
| Table 2: Distribution of patients across pathological findings of TEE. | |
| Pathological findings | N (%) |
| Wall motion abnormalities | 4 (2.8%) |
| Valve failure | 11 (7.7%) |
| Patent foramen ovale | 27 (18.9%) |
| Atrial septum aneurysm | 17 (11.9%) |
| ≤ 2 mm | 5 (3.5%) |
| 3 mm | 4 (2.8%) |
| 4 mm | 1 (0.7%) |
| Valsalva withdrawal | 16 (11.2%) |
| Thrombogenic aortic arch | 29 (20.3%) |
| 3rd degree | 1 (0.7%) |
| 4th degree | 6 (4.2%) |
| 5th degree | 22 (15.4%) |
| Aortic valve failure | 21 (14.7%) |
| 1st degree | 14 (9.8%) |
| 2nd degree | 6 (4.2%) |
| 3rd degree | 1 (0.7%) |
| Left atrial thrombus | 3 (2.1%) |
| Other thrombi | 6 (4.2%) |
| Table 3: Distribution of patients across antithrombotic treatment before and after TEE. | ||
| Medication | Before TEE | After TEE |
| N (%) | N (%) | |
| Antithrombotic therapy | 43 (30.1%) | 115 (80.4%) |
| Oral anticoagulants | 4 (2.8%) | 39 (27.3%) |
Figure 1: Transition in antithrombotic therapy.
Our study showed that TEE reveals a remarkable number of pathological findings and can lead to adjustment in antithrombotic therapy, including the administration of antiplatelet therapy in general, and oral anticoagulants (OACs/DOACs) specifically. This result is in line with the findings of other studies that have investigated the diagnostic impact of TEE in unselected patients with acute cerebral ischemia [28-30]. On the other hand our findings are in contrast to other studies. Using a retrospective chart review Menon, et al. [31] revealed that echocardiography detected potential clinically relevant findings in a minority of patients (7.6%), but these findings lead to a numerous change in medical management (90.5%).
Although LAT can be identified by TTE, the sensitivity of this procedure is low. Because of the left arterial location immediately adjacent to the esophagus, TEE is considered to be the gold-standard technique for detecting left atrial thrombi, with values of sensitivity and specificity approaching 99% [32]. However, the rate of TEE in the cohort in the present study was low, occurring in just 1 out of 10 stroke patients. In a multicenter cohort study of nearly 2.000 patients with ischemic stroke or TIA, 68% had an echocardiogram (most often a transthoracic), and the results were normal 86% of the time, whereas the 2 most common clinically actionable findings for secondary stroke prevention were cardiac thrombus and PFO [33]. The wide range of estimates for how often “clinically relevant” findings are detected on echocardiogram for patients with an ischemic stroke is partially related to changing criteria for what is considered clinically relevant, e.g., anticoagulation is no longer recommended in clinical practice guidelines for dilated cardiomyopathy [34]. While practice patterns change, updated data are necassery to re-evaluate the value of echocardiography. Once these data are available, there might be lags in changing clinical patterns until practice guidelines are updated.
Patent foramen ovale with an atrial septum aneurysm leads to an exponentially high risk of stroke [35]. In our study, we found a PFO prevalence of 18.9%. Recent studies have found that occlusion of a PFO might be associated with a reduced risk of subsequent stroke and recent clinical practice guidelines strongly recommend PFO closure for patients with cryptogenic stroke [15-17,36,37], but until 2018 PFO was considered to be an incidental finding [38], which likely is an explanation why none of our study patients with PFO were referred for closure of the defect. Given the large number of stroke patients who do not undergo TEE evaluation, there could presumably be a high number of unreported cases. A recent meta-analysis of prospective studies that compared the value of transthoracic to transesophageal echocardiography to detect PFO in patients with cryptogenic stroke determined the sensitivity of transthoracic echocardiography to be 45% (95% CI 31-60%) [39]. In the last 3 decades, the use of TEE in patients with stroke of uncertain etiology has revealed atherosclerotic plaques in the aortic arch, which often protrude into the lumen and have mobile components [40]. However, the Study of Perfusion and Anatomy`s Role in Coronary Artery (SPARC) study, conducted in a random population of patients at high risk of vascular events for whom TEE data were obtained, did not find an association between aortic atherosclerotic plaques and future cardiac or cerebrovascular events [41]. It is unknown what percentage of patients in routine care with cryptogenic stroke should ideally have a transesophageal echocardiogram, which is virtually 100% sensitive for PFO [39,42]. In the recent clinical trials for closure of PFO, patients had both a transthoracic and a transesophageal echocardiogram [15-17]. The PFO prevalence of 18.9% in our study may indicate the need for knowledge translation so that transesophageal echocardiography is considered for patients with cryptogenic stroke.
Since the Factor VII Activating Protease study (FSAPS) and other prospective case-control and follow-up studies, the 2 main, accepted criteria for embolic risk associated with aortic arch atheromas have been a plaque thickness ≥4 mm and the presence of mobile components [43-45], especially in combination with prothrombotic risk factors like hypercoagulability, antiphospholipid syndrome, protein C/S disorders, and vasculitis [46]. Aortic arch atheroma in young adults is rare, but its incidence and severity increases with age [47]. Thromboembolic events are associated with complex and ulcerated atherosclerotic plaques to which the thrombus is attached [48]. Previous studies have revealed associations between thromboembolic events and smoking, hypercholesterolemia, hypertension, diabetes, the male sex, and elevated plasma levels of fibrinogen and homocysteine [49,50].
In a retrospective study of patients who had suffered from a first cerebrovascular ischemic event [TIA or stroke], Young and Benesch found a high-risk source of embolus in 14.3% of patients, and an associated change in clinical management (including medication changes or subsequent testing) [51]. Furthermore, they found that an increased age and no history of diabetes mellitus were independently associated with a high-risk source of embolus. In our prospective study, we did not include patients with TIA. This might explain our finding of lower percentages of thrombi compared with those reported by Young and Benesch. Transient ischemic attacks may arise from different mechanisms than stroke. Therefore, it has been suggested that the inclusion of patients with TIA in a stroke trial could result in less-reliable findings.
Data from several prospective studies suggest that atheromatosis is a dynamic process; although plaque regression is possible, the most likely clinical course is slow progression [52-54]. Owing to routine exploration using TEE after supposed embolic events, reliable detection of mobile thoracic aorta thrombi has become more common [55]. Up to 40% of cerebral ischemic events are due to embolic causes. Further insight into cardioembolic etiology was expected from the embolic stroke of undetermined source (ESUS) approach. The two ESUS studies showed no benefit of anticoagulation over antiplatelet [56,57].
In our study, 57% of stroke patients were found to have abnormal TEE findings. Data regarding adjustment of medication due to TEE findings in stroke patients are sparse. In the study by Young and Benesch the results of the TEE evaluation lead to altered medication or clinical management in 30.3% of patients [51]. This is in contrast to our study, where 50% of the patients were started with antithrombotic therapy following TEE, especially oral anticoagulation in 24%. In addition, the average period of time that elapses between a patient’s admission to the hospital and TEE investigation is largely unknown. In this study, it was 4 days. Given a mean residence time of stroke patients in hospital of 11 days, in the evaluation period TEE was carried out within the first half of the hospital stay.
Our study has several limitations. First, we investigated a selected amount of stroke patients, infact those with presumed cardioembolic and cryptogenic etiology who were able to tolerate the invasive examination of TEE. Cardioembolic stroke often leads to severe constraints in patients that might avoid the procedure of TEE, so there is a lack of data from these patients. The amount of relevant findings might have been different, if we were able to investigate all cardioembolic stroke patients. Second, our study included only stroke patients admitted to hospital, and thus we may have overestimated the prevalence of echocardiographic abnormalities owing to the fact, that patients in hospital might suffer from a higher burden of comorbid conditions or more severe stroke compared with patients managed as outpatients. Third we identified patients with stroke by their main discharge diagnosis. Although the diagnostic codes have been validated, we might have missed patients who presented with stroke-like symptoms but were subsequently given a different diagnosis. Fourth, changing treatment is not a clinically relevant endpoint. Fifth, in patients with present atrial fibrillation by the time of admission further echocardiography investigations are often waived. For ongoing research selection criteria should be designated so that the results can be applied. It is possible that elderly patients are underrepresented in the cohort. The study did not include long-term follow up of the cohort, so it remains unclear if there is any evidence that the changes in treatment will lead to improved outcome. We did not account for complications in the TEE investigation; the sample size was too small for logistic regression analysis. The study does not compare findings in transthoracic with those in transesophageal echocardiogram, moreover we did not investigate any reasons why some patients underwent both procedures and some only TEE. Nonetheless, from the findings of this study we conclude that TEE evaluation is needed for the accurate assessment of ischemic stroke.
In our monocenter study, we found that transesophageal echocardiograms were normal in 43% of patients with cardioembolic and cryptogenic ischemic stroke. In contrast, the importance of ruling out clinically actionable findings in TEE for secondary stroke prevention, e.g., PFO in 18.9% of our study patients has taken on new importance, as closure of the defect reduces the risk of subsequent stroke by more than 50%.
Informed consent statement
The study was part of a benchmarking project (Quality of Treatment of Stroke in the Federal State Schleswig Holstein). The study was approved by the local ethics committee at the University of Lübeck. Each patient who participated in the study was required to sign an informed consent form that provided much more details on the study requirements.
- Bogousslavsky J, Kaste M, Skyhoj Olsen T, Hacke W, Orgogozo JM. Risk factors and stroke prevention. European stroke initiative (eusi). Cerebrovasc Dis. 2000;10 Suppl 3:12-21. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10940666
- Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: The german stroke data bank. Stroke 2001; 32: 2559-2566. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11692017
- Grau AJ, Eicke M, Biegler MK, Faldum A, Bamberg C, et al. Quality monitoring of acute stroke care in rhineland-palatinate, germany, 2001-2006. Stroke. 2010; 41: 1495-1500. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20522811
- Ferro JM. Cardioembolic stroke: An update. Lancet Neurol. 2003; 2: 177-188. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12849239
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the american heart association/american stroke association council on stroke: Co-sponsored by the council on cardiovascular radiology and intervention: The american academy of neurology affirms the value of this guideline. Stroke. 2006; 37: 577-617. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16432246
- Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, et al. Primary prevention of ischemic stroke: A guideline from the american heart association/american stroke association stroke council: Cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary working group; cardiovascular nursing council; clinical cardiology council; nutrition, physical activity, and metabolism council; and the quality of care and outcomes research interdisciplinary working group: The american academy of neurology affirms the value of this guideline. Stroke. 2006; 37: 1583-1633. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16675728
- Kittner SJ, Sharkness CM, Price TR, Plotnick GD, Dambrosia JM, et al. Infarcts with a cardiac source of embolism in the nincds stroke data bank: Historical features. Neurology. 1990; 40: 281-284. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2300250
- Hart RG. Cardiogenic embolism to the brain. Lancet. 1992; 339: 589-594. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3510609
- Ahmed N, Audebert H, Turc G, Cordonnier C, Christensen H, et al. Consensus statements and recommendations from the eso- karolinska stroke update conference, stockholm 11–13 November 2018. Eur Stroke J. 2019.
- Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European association of echocardiography (eae) (a registered branch of the esc). Eur J Echocardiogr. 2010; 11: 461-476. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20702884
- Yu EH, Lungu C, Kanner RM, Libman RB. The use of diagnostic tests in patients with acute ischemic stroke. Journal of stroke and cerebrovascular diseases: The official journal of National Stroke Association. 2009; 18: 178-184.
- Katsanos AH, Giannopoulos S, Frogoudaki A, Vrettou AR, Ikonomidis I, et al. The diagnostic yield of transesophageal echocardiography in patients with cryptogenic cerebral ischaemia: A meta-analysis. Eur J Neurol. 2016; 23: 569-579. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26918744
- Zhang L, Harrison JK, Goldstein LB. Echocardiography for the detection of cardiac sources of embolism in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis. 2012; 21: 577-582. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21367623
- Wolber T, Maeder M, Atefy R, Bluzaite I, Blank R, et al. Should routine echocardiography be performed in all patients with stroke? J Stroke Cerebrovasc Dis. 2007; 16: 1-7. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17689384
- Sondergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017; 377: 1033-1042. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28902580
- Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017; 377: 1022-1032. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28902590
- Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, et al. Patent foramen ovale closure or anticoagulation vs. Antiplatelets after stroke. N Engl J Med. 2017; 377: 1011-1021. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28902593
- Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr. 2016; 29: 1-42. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26765302
- Vitebskiy S, Fox K, Hoit BD. Routine transesophageal echocardiography for the evaluation of cerebral emboli in elderly patients. Echocardiography. 2005; 22: 770-774. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16194171
- de Bruijn SF, Agema WR, Lammers GJ, van der Wall EE, Wolterbeek R, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006; 37: 2531-2534. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16946152
- Leung DY, Black IW, Cranney GB, Walsh WF, Grimm RA, et al. Selection of patients for transesophageal echocardiography after stroke and systemic embolic events. Role of transthoracic echocardiography. Stroke. 1995; 26: 1820-1824. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7570732
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke. 2006; 37: 859-864. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16439702
- Cho HJ, Choi HY, Kim YD, Nam HS, Han SW, et al. Transoesophageal echocardiography in patients with acute stroke with sinus rhythm and no cardiac disease history. J Neurol Neurosurg Psychiatry. 2010; 81: 412-415. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19965855
- McGrath ER, Paikin JS, Motlagh B, Salehian O, Kapral MK, et al. Transesophageal echocardiography in patients with cryptogenic ischemic stroke: A systematic review. Am Heart J. 2014; 168: 706-712. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25440799
- Haeusler KG, Wollboldt C, Bentheim LZ, Herm J, Jager S, et al. Feasibility and diagnostic value of cardiovascular magnetic resonance imaging after acute ischemic stroke of undetermined origin. Stroke. 2017; 48: 1241-1247. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28411261
- Galougahi KK, Stewart T, Choong CY, Storey CE, Yates M, et al. The utility of transoesophageal echocardiography to determine management in suspected embolic stroke. Intern Med J. 2010; 40: 813-818. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19849751
- Ahmad O, Ahmad KE, Dear KB, Harvey I, Hughes A, et al. Echocardiography in the detection of cardioembolism in a stroke population. J Clin Neurosci. 2010; 17: 561-565. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20207149
- Pallesen LP, Ragaller M, Kepplinger J, Barlinn K, Zerna C, et al. Diagnostic impact of transesophageal echocardiography in patients with acute cerebral ischemia. Echocardiography. 2016; 33: 555-561. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27103483
- Censori B, Colombo F, Valsecchi MG, Clivati L, Zonca A, et al. Early transoesophageal echocardiography in cryptogenic and lacunar stroke and transient ischaemic attack. J Neurol Neurosurg Psychiatry. 1998; 64: 624-627. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9598678
- Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992; 326: 221-225. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1727976
- Menon BK, Coulter JI, Bal S, Godzwon C, Weeks S, et al. Acute ischaemic stroke or transient ischaemic attack and the need for inpatient echocardiography. Postgrad Med J. 2014; 90: 434-438. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24920609
- Nakanishi K, Homma S. Role of echocardiography in patients with stroke. J Cardiol. 2016; 68: 91-99. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27256218
- Fralick M, Goldberg N, Rohailla S, Guo Y, Burke MJ, et al. Value of routine echocardiography in the management of stroke. CMAJ. 2019; 191: E853-E859. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6682481/
- Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, et al. Acc/aha guidelines for the clinical application of echocardiography. A report of the american college of cardiology/american heart association task force on practice guidelines (committee on clinical application of echocardiography). Developed in collaboration with the american society of echocardiography. Circulation. 1997; 95: 1686-1744. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9118558
- Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001; 345: 1740-1746. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11742048
- Kuijpers T, Spencer FA, Siemieniuk RAC, Vandvik PO, Otto CM, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation therapy alone for management of cryptogenic stroke? A clinical practice guideline. BMJ. 2018; 362: k2515. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30045912
- Mir H, Siemieniuk RAC, Ge L, Foroutan F, Fralick M, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation in patients with patent foramen ovale and cryptogenic stroke: A systematic review and network meta-analysis incorporating complementary external evidence. BMJ Open. 2018; 8: e023761. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30121619
- Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992; 117: 461-465. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1503349
- Katsanos AH, Bhole R, Frogoudaki A, Giannopoulos S, Goyal N, et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology. 2016; 87: 988-995. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27488602
- Capmany RP, Ibanez MO, Pesquer XJ. Complex atheromatosis of the aortic arch in cerebral infarction. Curr Cardiol Rev. 2010; 6: 184-193. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21804777
- Meissner I, Khandheria BK, Sheps SG, Schwartz GL, Wiebers DO, et al. Atherosclerosis of the aorta: Risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiography study. J Am Coll Cardiol. 2004; 44: 1018-1024. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15337213
- Mojadidi MK, Winoker JS, Roberts SC, Msaouel P, Zaman MO, et al. Accuracy of conventional transthoracic echocardiography for the diagnosis of intracardiac right-to-left shunt: A meta-analysis of prospective studies. Echocardiography. 2014; 31: 1036-1048. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24689727
- Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994; 331: 1474-1479. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7969297
- French Study of Aortic Plaques in Stroke G, Amarenco P, Cohen A, Hommel M, Moulin T, Leys D, et al. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996; 334: 1216-1221. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8606716
- Pujadas R, Arboix A, Anguera N, Oliveres M, Massons J, et al. [role of complex aortic atheroma plaques in the recurrence of unexplained cerebral infarction]. Rev Esp Cardiol. 2005; 58: 34-40. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15680129
- Fernandez-Ortiz A, Badimon JJ, Falk E, Fuster V, Meyer B, et al. Characterization of the relative thrombogenicity of atherosclerotic plaque components: Implications for consequences of plaque rupture. J Am Coll Cardiol. 1994; 23: 1562-1569. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8195515
- Davila-Roman VG, Barzilai B, Wareing TH, Murphy SF, Schechtman KB, et al. Atherosclerosis of the ascending aorta. Prevalence and role as an independent predictor of cerebrovascular events in cardiac patients. Stroke. 1994; 25: 2010-2016. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8091446
- Laperche T, Laurian C, Roudaut R, Steg PG. Mobile thromboses of the aortic arch without aortic debris. A transesophageal echocardiographic finding associated with unexplained arterial embolism. The filiale echocardiographie de la societe francaise de cardiologie. Circulation. 1997; 96: 288-294. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9236447
- Tribouilloy C, Peltier M, Colas L, Senni M, Ganry O, et al. Fibrinogen is an independent marker for thoracic aortic atherosclerosis. The Am J Cardiol. 1998; 81: 321-326. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9468075
- Tribouilloy CM, Peltier M, Iannetta Peltier MC, Trojette F, Andrejak M, et al. Plasma homocysteine and severity of thoracic aortic atherosclerosis. Chest. 2000; 118: 1685-1689. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11115459
- Young KC, Benesch CG. Transesophageal echocardiography screening in subjects with a first cerebrovascular ischemic event. J Stroke Cerebrovasc Dis. 2011; 20: 503-509. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2997382/
- Sen S, Oppenheimer SM, Lima J, Cohen B. Risk factors for progression of aortic atheroma in stroke and transient ischemic attack patients. Stroke. 2002; 33: 930-935. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11935039
- Montgomery DH, Ververis JJ, McGorisk G, Frohwein S, Martin RP, et al. Natural history of severe atheromatous disease of the thoracic aorta: A transesophageal echocardiographic study. J Am Coll Cardiol. 1996; 27: 95-101. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8522717
- Geraci A, Weinberger J. Natural history of aortic arch atherosclerotic plaque. Neurology. 2000; 54: 749-751. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10680818
- Davila-Roman VG, Westerhausen D, Hopkins WE, Sicard GA, Barzilai B. Transesophageal echocardiography in the detection of cardiovascular sources of peripheral vascular embolism. Ann Vasc Surg. 1995; 9: 252-260. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7632553
- Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018; 378: 2191-2201. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29766772
- Diener Hea. Re-spect esus: Dabigatran versus acetylsalicylic acid for stroke prevention in patients with embolic stroke of undetermined source. Presented at the world stroke congress, montreal, canada, on 17 october 2018. 2018.