More Information
Submitted: 19 February 2020 | Approved: 02 March 2020 | Published: 03 March 2020
How to cite this article: Ujuanbi AS, Onyeka CA, Yeibake WS, Oremodu T, Kunle-Olowu OE, et al. Pathological left ventricular hypertrophy and outflow tract obstruction in an infant of a diabetic mother: A case report. J Cardiol Cardiovasc Med. 2020; 5: 047-050.
DOI: 10.29328/journal.jccm.1001085
ORCiD: orcid.org/0000-0002-5222-487X
Copyright License: © 2020 Ujuanbi AS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pathological left ventricular hypertrophy; Outflow tract obstruction; Infant of diabetic mother
Pathological left ventricular hypertrophy and outflow tract obstruction in an infant of a diabetic mother: A case report
Ujuanbi AS1*, Onyeka CA1, Yeibake WS1, Oremodu T1, Kunle-Olowu OE2 and Otaigbe BE3
1Department of Paediatrics, Federal Medical Centre, Yenagoa, Bayelsa, Nigeria
2Department of Paediatrics, Niger Delta University Teaching Hospital, Bayelsa, Nigeria
3Department of Paediatrics, University of Port Harcourt Teaching Hospital, Port Harcourt, Rivers State, Bayelsa, Nigeria
*Address for Correspondence: Ujuanbi Amenawon Susan, Department of Paediatrics, Federal Medical Centre, Yenagoa, Bayelsa, Nigeria, Tel: +2348061379136; Email: [email protected]
Background: Infants of diabetic mothers (IDMs) are at increased risk of developing congenital anomalies including cardiac defects. Pathological left ventricular hypertrophy, asymmetrical septal hypertrophy and outflow tract obstruction is a rare but known cardiac comorbidity in infants of diabetic mothers. The severity of this condition in IDMs can vary from an incidental finding on echocardiography to an infant with severe symptoms of congestive heart failure and specific management of the condition varies.
Aim: The aim of this article is to report this clinical entity in a Nigerian infant born to a mother with poor glycaemic control in pregnancy and highlight management.
Case report: We report a term neonate who was diagnosed as a case of pathological left ventricular hypertrophy, asymmetrical septal hypertrophy and outflow tract obstruction delivered to a mother with gestational diabetics with poor glycaemic control in pregnancy. Child was treated successfully with β-adrenergic blocker and showed resolution of hypertrophy in follow-up echocardiography.
Conclusion: Infants of diabetic mothers are very high risk infants. Pathological left ventricular hypertrophy in IDM have good prognosis. Early recognition and prompt intervention is advocated.
Globally, the incidence of diabetes mellitus including diabetes in pregnant women is on the increase [1]. Diabetes mellitus in a pregnant woman jeopardizes not only maternal health but can also have significant implications on the child to be born [1,2].
Despite the recent advances in perinatal care around the world, infants of diabetic mothers (IDMs) remain at risk of multiple problems in the perinatal period that includes various metabolic, physiologic and congenital complications including preterm birth, birth asphyxia, macrosomia, respiratory distress including hyaline membrane disease and transient tachypnea of newborn (TTNB), hypoglycemia, hypocalcemia, hyperbilirubinemia, polycythemia and hyperviscosity syndrome and congenital anomalies, particularly of the central nervous system and cardiovascular system [2-4]. Cardiac complications due to congenital heart malformation and pathological left ventricular hypertrophy and outflow tract obstruction are the major causes of morbidity and mortality in fetuses and newborns of mothers with IDM [5,6].
Poor maternal glycemic control is associated with neonatal biventricular hypertrophy that may occasionally be associated with left ventricular outflow tract (LVOT) obstruction because of marked interventricular septal hypertrophy [7]. Five percent to 10% of these infants may develop impaired cardiac output because of LVOT obstruction and diminished ventricular volumes [8]. Spontaneous regression of hypertrophy may occur as plasma insulin concentrations normalize within the first few months of life [7-10]. As there is no specific treatment plan for infant of diabetic mothers with pathological left ventricular hypertrophy, asymmetrical septal hypertrophy and outflow tract obstruction with cardiac dysfunction, the use of β-adrenergic blockers have been found to be useful. We report a case of a Nigerian neonate that was diagnosed with pathological left ventricular hypertrophy, asymmetrical septal hypertrophy and outflow tract obstruction and dynamic subaortic stenosis who was born to a mother with gestational diabetics who had poor glycemic control. Child was managed with β-adrenergic blocker and showed resolution of hypertrophy in follow-up echocardiography reviews.
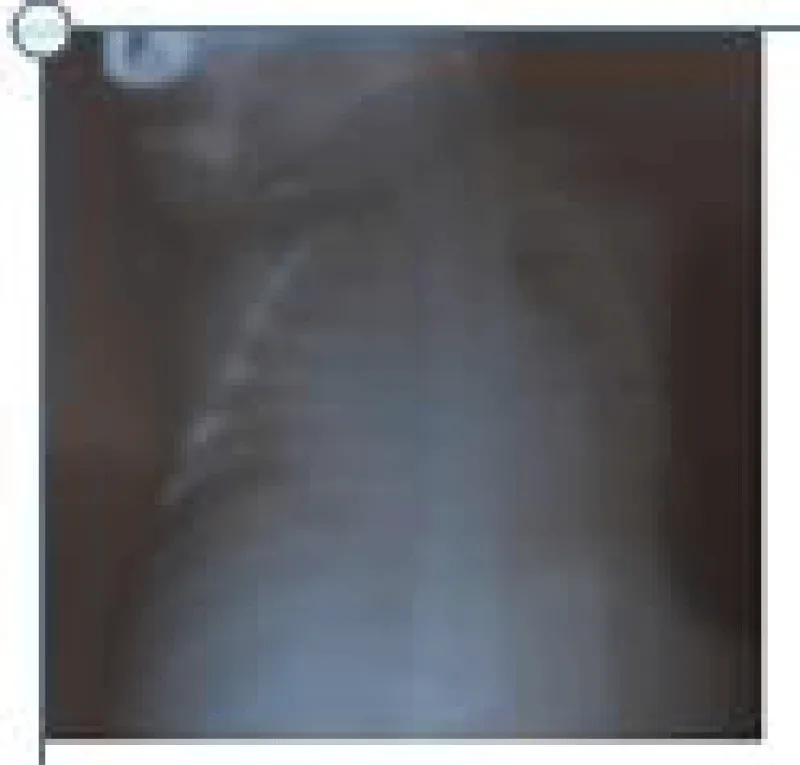
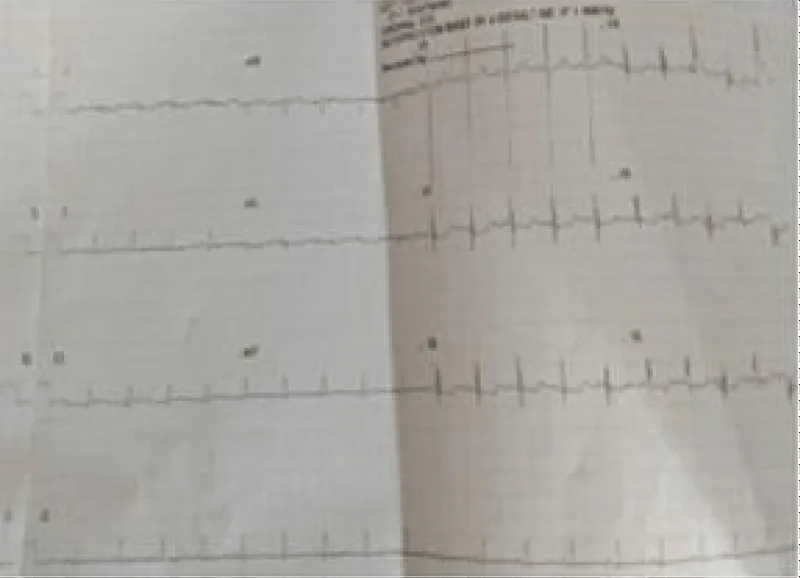
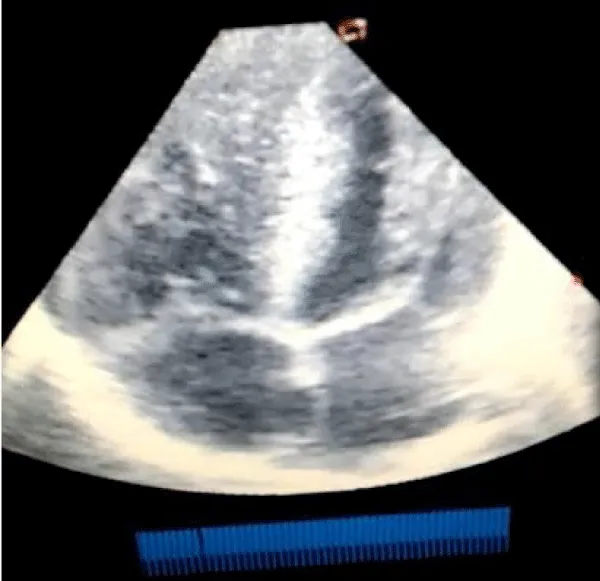
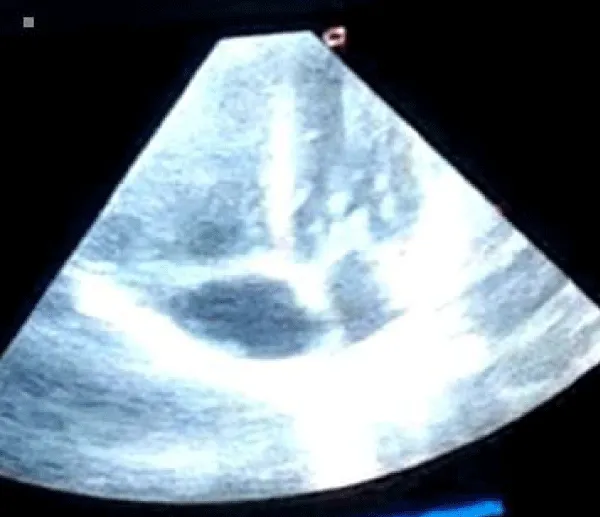
A male neonate delivered at term via SVD to a 24 years old G2P2 mother, who was diagnosed with gestational diabetes at 31 weeks of gestation who had poor glycemic control despite being on subcutaneous insulin and dietary modifications. Mother also had gestational diabetics during her first pregnancy (deranged glycated haemoglobin HbA1c) and claimed her blood sugar normalized after delivery. Her first child was a female and weighed 4.5 kg, alive and well and did not have any immediate neonatal complications. No family history of cardiac diseases. Birth weight of index baby was 4.8 kg (> 95% centile for age) and Apgar scores were 5/7/7 in 1, 5 and 10 minutes respectively. The physical examination showed a macrosomic infant (weight > 95% centile for age), thick upper extremity skin folds, broad shoulder and torso with a relatively smaller head, and plethoric look. He had respiratory distress with peripheral cyanosis. Oxygen saturation (SPO2) was 92% (room air) and urgent random blood sugar (RBS) was 4.6mmol/l. There were no other obvious malformations. Baby was admitted immediately after birth into the special care baby unit (SCBU) of a Federal Medical Centre in southern part of Nigeria for moderate birth asphyxia, severe respiratory distress and macrosomia. Child had persistent respiratory distress while on admission and further cardiac evaluation revealed displaced apex beat with no cardiac murmur. Chest x-ray done showed severe cardiomegaly with bi-ventricular and right atrial enlargements and a cardiothoracic ratio of 68% and oligaemia (Figure 1). Electrocardiography showed evidence of biventricular hypertrophy, right atrial enlargement, sinus tachycardia and occasional premature ventricular complexes (Figure 2). Echocardiography done on the 7th day of life showed severe bi-ventricular hypertrophy with significant narrowing of the left ventricular cavity, marked asymmetrical septal hypertrophy, moderate to severe dynamic subaortic stenosis (Peak gradient 45 mmHg) and left ventricular systolic and diastolic dysfunction (Figure 3). He was commenced on tabs propranolol at 1mg/kg/dose 8hrly and discharged after 5 days with improvement in respiratory distress. He also received oxygen therapy, intravenous fluid and intravenous antibiotics as well as intensive nursing care while on admission. Follow up echocardiography at 6 wks of life showed evidence of remarkable resolution of hypertrophy and improvement in ventricular function (Figure 4).
Figure 1: Chest X-Ray showing massive cardiomegaly (CTR = 68%).
Figure 2: Electrocardiography showing sinus tachycardia and biventricular hypertrophy.
Figure 3: Echocardiography (apical 4 chamber view) showing severe biventricular and septal hypertrophy causing left ventricular outflow tract obstruction and a markedly diminished left ventricular cavity.
Figure 4: Repeat Echocardiography at 6 weeks of life (apical 4 chamber view) showing significant improvement in ventricular wall and septal enlargement.
Pathological left ventricular hypertrophy, asymmetrical septal hypertrophy and outflow tract obstruction is a condition characterised by stiff, hypertrophied ventricular muscle, predominant thickening of the ventricular septum, impaired relaxation, and powerful but incoordinate contraction [11]. These findings were present in our infant as seen on the echocardiography. There is functional subaortic obstruction in severe cases and this is referred to as idiopathic hypertrophic subaortic stenosis (IHSS). Our infant had moderate to severe functional subaortic stenosis and other features of severe form of cardiomyopathy with asymmetrical septal hypertrophy.
There have been reports of hypertrophic cardiomyopathy in infancy [12-15] and either due to a positive family history of familial cardiomyopathy [15] or the presence of maternal diabetes [12-14] which seems to be a common association. In our infant, there was no family history of familial cardiomyopathy or sudden death although we did not perform echocardiographic studies on his 1st-degree relatives, rather his mother had recurrent gestational diabetes mellitus during pregnancy and the diabetes had been poorly controlled. This is similar to the observation of Halliday, et al. [16], in a study of twelve newborn infants with hypertrophic cardiomyopathy delivered to women with poorly controlled diabetic mellitus. Our infants had cardiomegaly on radiographic and echocardiographic examination. Comparable findings were reported by Howard, et al. [17], in study of symptomatic and asymptomatic infants of diabetic mothers with hypertrophic cardiomyopathy. In our infant the cardiomegaly was due to hypertrophy especially of the interventricular septum and left ventricular posterior wall.
There have been previous reports of associated congenital heart disease in infants of diabetic mothers with hypertrophic cardiomyopathy [17], our infant however did not have any congenital heart disease. Usually the infants are asymptomatic, but 5%-10% have respiratory distress or signs of poor cardiac output or heart failure [2]. Our infant was symptomatic and respiratory distress was the most common symptom necessitating assisted ventilation. The babies of diabetic mothers are typically large for gestational age and often have generalized organomegaly [17,18]. Our infant was macrosomic with a weight of 4.8 kg. Maternal hyperglycemia, with resultant fetal hyperglycemia, stimulates fetal islet cells and produces persistent fetal hyperinsulinemia, which may promote increased glycogensis, lipogensis and protein synthesis, with resultant obesity and macrosomia [18]. It is possible that the cardiac hypertrophy of IDMs represents another manifestation of their generalized organomegaly which was present in our infant.
The cardiomyopathy in infants of diabetic mother is transient and this property makes it distinctive over other forms of cardiomyopathy. It sometimes resolve with normalization of plasma insulin levels [7-10]. Our patient was symptomatic and therefore needed immediate supportive care. The affected infants usually recover within 2 to 3 weeks of supportive care, and echocardiographic findings shows normalization within 6-12 months [2] as demonstrated in our infant. Supportive care includes increased intravenous fluid administration and beta blockers like propranolol. Our infant improved with propranolol administration and intensive supportive care and was discharged home in a stable clinical state. Digitalis and other positive inotropic agents like dobutamine and dopamine are contraindicated in such infants as they increase the risk of ventricular outflow tract obstruction which can worsen their cardiac function [19].
Pathological left ventricular hypertrophy, and outflow tract obstruction of IDM have good prognosis. Early echocardiography, close monitoring and regular follow-up are advocated. Beta blockers like propranolol can be started if the HOCM is symptomatic.
- Venkat Rk, Aakash P, Anish P. Infant of diabetic mother: what one needs to know? J Matern Fetal Neonatal Med. 2020; 33: 482-492. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29947269
- Sharma D, Pandita A, Shastri S, Sharma P. Asymmetrical septal hypertrophy and hypertrophic cardiomyopathy infant of diabetic mother: a reversible cardiomyopathy. Med J. 2016; 9: 257-260.
- Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med. 1994; 45: 245-260. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8198381
- Russell NE, Holloway P, Quinn S, Foley M, Kelehan P, et al. Cardiomyopathy and cardiomegaly in stillborn infants of diabetic mothers. Pediatr Dev Pathol. 2008; 11: 10-14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18237240
- Demiroren K, Cam L, Oran B, Koç H, Başpinar O, et al. Echocardiographic measure-ments in infants of diabetic mothers and macrosomic infants of nondiabetic mothers. J Perinat Med. 2005; 33: 232-235. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15914346
- Park MK. Pediatric cardiology. 4th ed. St. Louis: Mosby; 2002. Primary myocardial disease. 2002; 267-392.
- Ullmo S, Vial Y, Di Bernardo S, Roth-Kleiner M, Mivelaz Y, et al. Pathologic ventricular hypertrophy in the offspring of diabetic mothers: a retrospective study. Eur Heart J. 2007; 28: 1319–1325. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17158827
- Hay WW. Care of the infant of the diabetic mother. Curr Diab Rep. 2012; 12: 4–15. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22094826
- Reller MD, Kaplan S. Hypertrophic cardiomyopathy in infants of diabetic mothers: an update. Am J Perinatol. 1988; 5: 353-358. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2971360
- Way GL, Wolfe RR, Eshaghpour E, Bender RL, Jaffe RB, et al. The natural history of hypertrophic cardiomyopathy in infants of diabetic mothers. J Pediatr. 1979; 95: 1020-1025. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/159352
- Goodwin JF. A current appraisal of the cardiomyopathies. Hosp Update. 1979; 5: 665-683
- Poland R L, Walther LJ, Chang L. Hypertrophic cardiomyopathy in infants of diabetic mothers (abstract). Pediatr Res. 1975; 9: 269.
- Way GL, Ruttenberg HD, Eshaghpour E, Nora JJ, Wolfe RE. Hypertrophic obstructive cardiomyopathy in infants of diabetic mothers (abstract). Circulation. 1976; 53-54.
- Gutgesell HP, Mullins CE, Gillette PC, Speer M, Rudolph AJ, et al. Transient hypertrophic subaortic stenosis in infants of diabetic mothers. J Pediatr. 1976; 89: 120-125. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/945337
- Maron BJ, Edwards JE, Henry WL, Clark CE, Bingle GJ, et al. Asymmetric septal hypertrophy (ASH) in infancy. Circulation. 1974; 50: 809-820. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4278818
- Halliday HL. Hypertrophic cardiomyopathy in infants of poor1y-controlled diabetic mothers. Arch Dis Childhood. 1981; 56: 258-263.
- Howard PG, Michael ES, Harvey SR. Characterization of the Cardiomyopathy in Infants of Diabetic Mothers. Circulation. 1980; 61: 441-450. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6444280
- Bulkley BH, Weisfeldt ML, Hutchins GM. Isometric cardiac contraction. a possible cause of the disorganized myocardial pattern of idiopathic hypertrophic subaortic stenosis. N Engl J Med. 1977; 296: 135-139. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/556638
- Denfield SW, Gajarski RJ, Towbin JA. Cardiomyopathies. In: Garson AG, Bricker JT, Fisher DJ, Neish SR, editors. The Science and Practice of Pediatric Cardiology. 1998; 1851.