More Information
Submitted: 15 March 2020 | Approved: 02 April 2020 | Published: 03 April 2020
How to cite this article: Toquero J, Durante-López A, González-Mirelis J, Castro V, García-Izquierdo E, et al.. Recurrence of atrial fibrillation after pulmonary vein isolation, should we change the energy and technique? J Cardiol Cardiovasc Med. 2020; 5: 073-079.
DOI: 10.29328/journal.jccm.1001090
ORCiD: orcid.org/0000-0002-9549-9064
Copyright License: © 2020 Toquero J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Atrial fibrillation ablation; Pulmonary vein isolation; Cryoablation; Radiofrequency; Crossover strategy
Recurrence of atrial fibrillation after pulmonary vein isolation, should we change the energy and technique?
Jorge Toquero Ramos*, Alejandro Durante-López, Jesús González Mirelis, Víctor Castro Urda, Eusebio García Izquierdo, Diego Jiménez Sánchez and Ignacio Fernández-Lozano
Cardiology Department. Puerta de Hierro Hospital, Madrid, Spain
*Address for Correspondence: Jorge Toquero Ramos, Puerta de Hierro Hospital. Cardiology Department, Manuel de Falla, 1. 28220. Majadahonda Madrid, Spain, Tel: +34 630916248; Email: [email protected]
Background: Pulmonary vein isolation (PVI) is the accepted standard nowadays for atrial fibrillation (AF) ablation. The most widespread ablation techniques are cryoballoon (CB) and point-by-point radiofrequency (RF) ablation. Comparative studies between both techniques have shown their equivalence for the first ablation procedure, but no trial has explored the potential incremental benefit of crossing over the ablation technique after AF recurrence.
Objective: To explore the potential incremental benefit of a crossover ablation strategy for AF recurrences, comparatively with repeating the same ablation energy used for the first procedure.
Methods: Retrospective analysis of patients undergoing a second AF ablation procedure after documented AF recurrence. Patients were excluded if all 4 PV were isolated at the beginning of the second procedure or extra-PVI ablation was used for the second procedure. Crossover group (n = 16) included patients in which two different techniques were used for the first and second procedure (CB-RF or RF-CB). Control group (n = 23) for those with same ablation procedure (RF-RF of CB-CB). Acute procedure end-point was PVI of all four pulmonary veins. Patients were followed-up at 3, 6, and 12 months with an electrocardiogram and a 24 h-holter. Arrhythmia-free survival at 1 year after the second ablation procedure was studied, comparing efficiency and safety of the two approaches (crossover vs. same energy). Success was defined as freedom from AF or atrial tachycardia lasting > 30 s off antiarrhythmic drugs (AADs)
Results: A cohort of 39 paroxysmal and persistent AF patients was analyzed. PVI after the second procedure was 100% in all patients in both groups. There were no baseline relevant differences between the two groups. No deaths or hospitalizations occurred during follow up (data censored at 24h moths). At 1 year, arrhythmia free-survival was significantly higher in the crossover group compared to control group [93,3% vs. 47,8%; HR 0.19 (0.06-0.66);p = 0,009].
Conclusion: Crossing the ablation technique (point-by-point radiofrequency or cryoballoon PVI) after AF recurrence significantly improved arrhythmia free-survival at one year, despite no difference in acute success (PVI isolation). Randomized controlled trials with a higher amount of patients are needed to confirm the results and widespread this approach.
Atrial fibrillation (AF), the most frequent cardiac arrhythmia, is associated with increased risk of stroke and heart failure, in addition to a higher rate of mortality [1]. Sinus rhythm can often be restored with electric cardioversion; however, the rate of AF recurrence is high, even with administration of antiarrhythmic drugs (AADs) [2]. In addition to their relatively low efficacy, AADs have the disadvantage of causing adverse events, often leading to discontinuation [3,4]. Catheter ablation is the most successful technique to treat drug-refractory patients with atrial fibrillation [5] and persistent pulmonary vein isolation (PVI) is the cornerstone of the procedure. Two are the most widely accepted techniques for PVI: point-by-point radiofrequency (RF) ablation or cryoballoon ablation (CB). Several trials have proved their equivalence in terms of success and complications, and both are approved to be used in current practice [3-7].
However, about a third of patients had a recurrence during first year of follow-up after PVI independently of the technique used. No trial has evaluated so far the potential added value of crossing the ablation technique used for the first procedure, using cryoballoon in the second procedure if the first one was performed by point-by-point RF and the other way around (crossover strategy).
For patients with non-paroxysmal forms of atrial fibrillation, the pathophysiology is less known and variable among patients, so in an attempt to improve the results of ablation, in recent years much attention has been focused to the addition of new strategies to the PVI, such as mapping and ablation of rotational activity, ablation of low-voltage areas in the left atrium, ablation of areas of fibrosis previously identified with magnetic resonance studies, ablation of extra-pulmonary foci or ablation or ligature of the left appendage. However, none of these strategies has shown superiority to the electrical PVI in a first ablation procedure of atrial fibrillation [8]. As a result, ensuring durable PVI is still the recommended approach, even for a second ablation procedure.
The main objective of our study was to demonstrate that, in patients with an indication for a second ablation procedure due to AF recurrence, PVI by means of a crossover strategy might improve long-term efficacy and reduce further recurrences.
Patients
We performed a retrospective analysis of patients undergoing a second AF ablation procedure after documented AF recurrence. An image test (cardiac MR or CT scan) preablation was performed on every patient to assess pulmonary vein distribution and size, left atrial size and esophageal position.
From our cohort we excluded those patients in which all 4 PV were isolated at the beginning of the second procedure or extra-PVI ablation was used for the second procedure. 39 consecutive patients, after exclusions, were finally analyzed. The Institutional Committee on Human Research approved the study and patient information was de-identified.
Crossover group comprised patients in which the ablation energy used for the second ablation procedure was changed compared to the first one, regardless of the order they were used (RF-CB or CB-RF). Control group included those patients for whom the second procedure used the same energy and approach than the first one (RF-RF or CB-CB).
The ablation technique for the first and the second procedure were left to the treating physician discretion, based mostly, but not exclusively, on anatomical parameters from the image test (common trunk, presence of supernumerary veins or pulmonary vein size). Also, the pre-ablation probability of extra-PVI ablation was taken into account, based on left atrial diameter and volume, low LVEF or previous cardiac surgery.
By reviewing the ablation and procedure data recorded from the first procedure, treating physician decided to use the same energy for the second procedure (when nothing suspicious of potential gap for RF or very late isolation for CB) or to change the energy otherwise. Patients were excluded if all 4 PV were isolated at the beginning of the second procedure or extra-PVI ablation was used for the second procedure. Crossover group (n = 16) included patients in which two different techniques were used for the first and second procedure (CB-RF or RF-CB). Control group (n = 23) for those with same ablation procedure (RF-RF of CB-CB). Acute procedure end-point was PVI of all four pulmonary veins.
Patients treated since 2010 were studied and, due to variability in years of follow-up, data were censored at 24 months. Patients were followed-up at 3, 6, and 12 months, including in-office symptoms evaluation, ECG and 24h-holter monitoring. Antiarrhythmic drugs were maintained during the 3-month blanking period, and at the treating physician discretion afterwards in case of symptoms. Arrhythmia-free survival at 1 year after the second ablation procedure was analyzed, comparing efficiency and safety of the two approaches (crossover vs. same ablation energy). Success was defined as freedom from AF or atrial tachycardia lasting > 30 s off antiarrhythmic drugs (AADs).
Pvi technique
Radio frequency: After double transseptal puncture to get access to the left atrium with a preshaped sheath (Abbott Swartz™ Braided SL™ Transseptal Guiding Introducer Sheath SL0) an electroanatomical map with Biosense CARTO mapping system was performed by means of a Circular multipolar catheter (Lasso®, 20 poles, Biosense Webster, Inc.). Ablation catheter was an irrigated force-control Thermocool Smarttouch® ablation catheter (Biosense Webster, Inc.). Ablation was performed point by point at PV antrum, with a target power of 30W at the posterior wall and 35W at the anterior wall. Ablation points were colour-coded by means of Visitag module (Carto Biosense Webster, Inc.) with a 10 ohm impedance decrease target on each point. Using the multipolar catheter signals inside the vein during the ablation procedure and pacing from inside the vein after completing isolation checked entrance and exit block for each vein.
Cryoablation: After single transseptal puncture with a preshaped sheath (Abbott Swartz™ Braided SL™ Transseptal Guiding Introducer Sheath SL0) this was exchanged to a Flexcath Advance™ steerable sheath. Arctic Front Advance™ Cardiac Cryoablation Catheter (cryo-balloon, 28 mm) was inserted, jointly with an Achieve or Achieve Advance™ Mapping Catheter (Medtronic, Inc.). The Achieve catheter was sequentially positioned in each vein, the cryo-balloon was advanced, the vein occluded, freezed and isolated. Cryo-dosing for the patients included in this analysis consisted on a first 4 min freeze followed by a bonus freeze of 3 min per vein. Entrance and exit block was checked for each and every vein.
Statistical analysis
Due to the non-randomized nature of our study, an initial descriptive analysis was performed comparing the four groups in the order the two techniques were used (RF-RF, CB-CB, RF-CB and CB-RF) looking for homogeneity and the potential influence of the technique used first. Later, the same descriptive analysis was performed by grouping the populations of interest (crossover vs. no-crossover group). Normality of data distribution was evaluated for each variable using Kolmogorov-Smirnov test. Data are expressed as frequencies and percentages or mean ± standard deviation (median and interquartile range for non-normally distributed variables). The chi-square test and Student´s unpaired two-tailed t-test were used to determine differences between groups. For nonparametric variables Fisher’s or Mann-Whitney test were used in two-group comparisons. Two-tailed p values <0.05 were considered significant.
Finally, a comparative analysis of AF recurrence post-second procedure was performed. After a blanking period of 3 months, AF recurrence was considered if any episode of AF greater than 30 seconds was documented by ECG or 24 h Holter. Kaplan-Meier was used to represent the recurrence of AF during follow-up. All analyses were carried out using SPSS version 20 (SPSS, Inc., Chicago, IL, USA).
Our cohort comprised 39 paroxysmal (n = 21) and persistent (n = 18) AF patients. Long-standing persistent AF patients were excluded. There were no statistically significant differences in the number of patients with incomplete isolation of the 4 veins at the end of the first procedure (Crossover group 4%, no crossover group 7%,p = 0.76). Also, there were no differences in the number of patients with at least one pulmonary vein reconnected at the beginning of the second procedure for AF recurrence (crossover group 93%, no crossover group 90%,p = 0.69). All veins were isolated after the second ablation procedure in 100% of the patient population, no matter the energy used.
As shown in tables 1,2, 21 patients were taken AAD before the second procedure (9 on amiodarone, 7 on group 1C AAD and 5 others: 3 dronedarone and 2 sotalol). AAD were maintained until the 3-month follow-up (blanking period), when removed or maintained according to the decision of the treating physician. Only 2 patients remained on AAF at the 12-month follow-up.
A first pre-established descriptive analysis was performed to verify homogeneity and comparability of the different patient groups (RF-RF, CB-CB, RF-CB and CB-RF; Table 1). 22 baseline clinical, pharmacological and anatomical characteristics were analyzed. Then we proceeded to perform a descriptive study of both groups of study (crossover N = 16 vs. no-crossover N = 23) (Table 2). There were a higher number of males both in the crossover (63%) and no-crossover groups (91%), but the difference was more striking in the no-crossover group, that explained the significantly p-value encountered (p = 0,03). Also left atrial volume index (LA VOLi), but not diameter, was higher in crossover group (LA VOLi > 40 ml/m2 44% vs. 13% respectively;p = 0,03). No baseline relevant differences on anatomical characteristics between the two groups were encountered, including number of veins, their conformation, maximum size, presence of common trunks or presence of supernumerary veins. Analogous to the sphericity index used to define the left ventricular shape, we defined an sphericity index (relation between mayor and minor diameter at ostia level for each pulmonary vein) to analyze the potential relationship between pulmonary vein shape and the acute (PVI) and long-term results (AF recurrence). There were no differences in sphericity index between four groups (CB-CB, RF-RF, CB-RF, RF-CB;p = 0,44) (Table 1) or in both groups of interest (no-crossover 1.18 ± 0.33 vs. crossover 1.26 ± 0.44,p = 0,34) (Table 2).
| Table 1: Patient baseline characteristics separated into four groups. CB-CB: Cryoballoon-Cryoballoon; RF-RF: Radiofrequency-Radiofrequency; CB-RF: Cryoballoon-Radiofrequency; RF-CB: Radiofrequency-Cryoballoon; | ||||||
| CB-CB | RF-RF | CB-RF | RF-CB | p - value | ||
| Sex | Male | 5 (100%) | 16 (89%) | 2 (29%) | 8 (89%) | < 0.01 |
| Female | 0 (0%) | 2 (11%) | 5 (71%) | 1(11%) | ||
| Age >65 | No | 5 (100%) | 12 (67%) | 4 (57%) | 6 (67%) | 0.43 |
| Yes | 0 (0%) | 6 (33%) | 3 (43%) | 3 (33%) | ||
| BMI >25 | No | 2 (40%) | 3 (17%) | 0 (0%) | 2 (22%) | 0.35 |
| Yes | 3 (60%) | 15 (83%) | 7 (100%) | 7 (78%) | ||
| HTA | No | 1 (20%) | 9 (50%) | 3 (50%) | 3 (33%) | 0.36 |
| Yes | 4 (80%) | 9 (50%) | 3 (50%) | 6 (67%) | ||
| Smoker | No | 2 (40%) | 8 (44%) | 6 (86%) | 6 (67%) | 0.22 |
| Yes | 3 (60%) | 10 (66%) | 1 (14%) | 3 (33%) | ||
| Previous Cardiac Surgery | No | 4 (80%) | 14 (78%) | 7 (100%) | 6 (66%) | 0.43 |
| Yes | 1 (20%) | 4 (22%) | 0 (0%) | 3 (33%) | ||
| Ischemic Heart Disease | No | 4 (80%) | 17 (94%) | 7 (100%) | 8 (89%) | 0.59 |
| Yes | 1 (20%) | 1 (6%) | 0 (0%) | 1 (11%) | ||
| Moderate-severe mitral valve disease | No | 5 (100%) | 18 (100%) | 7 (88%) | 9 (100%) | 0.20 |
| Yes | 0 (0%) | 0 (0%) | 1 (12%) | 0 (0%) | ||
| LVEF <55% | No | 5 (100%) | 16 (89%) | 6 (86%) | 8 (89%) | 0.87 |
| Yes | 0 (0%) | 2 (11%) | 1 (14%) | 1 (11%) | ||
| Beta-blockers | No | 1 (25%) | 8 (44%) | 1 (17%) | 6 (67%) | 0.17 |
| Yes | 3 (75%) | 10 (56%) | 5 (83%) | 3 (33%) | ||
| AAD | No | 2 (40%) | 11 (61%) | 2 (29%) | 3 (33%) | 0.37 |
| Yes | 3 (60%) | 7 (39%) | 5 (71%) | 6 (67%) | ||
| AAD group 1C | No | 4 (80%) | 15 (83%) | 5 (71%) | 8 (89%) | 0.84 |
| Yes | 1 (20%) | 3 (17%) | 2 (29%) | 1 (11%) | ||
| Amiodarone | No | 3 (60%) | 15 (83%) | 5 (71%) | 7 (78%) | 0.72 |
| Yes | 2 (40%) | 3 (17%) | 2 (29%) | 2 (22%) | ||
| OAC | No | 2 (40%) | 8 (44%) | 0 (0%) | 4 (44%) | 0.19 |
| Yes | 3 (60%) | 10 (56%) | 7 (100%) | 5 (56%) | ||
| NOAC | No | 4 (80%) | 12 (77%) | 5 (71%) | 8 (89%) | 0.64 |
| Yes | 1 (20%) | 6 (33%) | 2 (29%) | 1 (11%) | ||
| VKA | No | 3 (60%) | 14 (78%) | 2 (29%) | 5 (56%) | 0.15 |
| Yes | 2 (40%) | 4 (22%) | 5 (71%) | 4 (44%) | ||
| Type of AF | Paroxysmal | 3 (60%) | 9 (50%) | 3 (43%) | 6 (67%) | 0.13 |
| Persistent | 2 (40%) | 9 (50%) | 4 (57%) | 3 (33%) | ||
| LA diameter >45mm | No | 3 (60%) | 10 (66%) | 4 (57%) | 6 67%) | 0.96 |
| Yes | 2 (40%) | 8 (44%) | 3 (43%) | 3 (33%) | ||
| LA VOLi > 40ml/m2 | No | 5 (100%) | 15 (83%) | 3 (43%) | 6 (67%) | 0.09 |
| Yes | 0 (0%) | 3 (17%) | 4 (57%) | 3 (33%) | ||
| CHADS2 | 1 (0.36-1.64) | 0.89 (0.43-1.35) | 1 (0.38-1.62) | 1 (0.52-1.48) | 0.94 | |
| CHA2DS2-VASc | 1 (0.36-1.64) | 1.33 (0.75-1.91) | 2 (1.02-2.98) | 1.44 (0.62-2.26) | 0.54 | |
| Sphericity Index | 1.15 (0.91-1.39) | 1.20 (0,82-1,58) | 1.33 (0.89-1.77) | 1.17 (0.79-1.55) | 0.44 | |
| AF: Atrial Fibrillation; HTA: Hypertension; AAD: Anti-Arrhythmic Drugs; LA VOLi: Left Atrial Volume Index; NOAC: Non-Vitamin K Antagonist Oral Anticoagulant; OAC: oral anticoagulation, VKA: Vitamin K Antagonist. | ||||||
| Table 2: Patient baseline characteristics grouped in two groups: crossover and no-crossover. | ||||
| NO CROSSOVER | CROSSOVER | p - value | ||
| Sex | Male | 21 (91%) | 10 (63%) | 0.03 |
| Female | 2 (9%) | 6 (37%) | ||
| Age >65 | No | 17 (74%) | 10 (62%) | 0.60 |
| Yes | 6 (26%) | 6 (38%) | ||
| BMI >25 | No | 5 (22%) | 2 (13%) | 0.46 |
| Yes | 18 (78%) | 14 (87%) | ||
| HTA | No | 10 (43%) | 6 (40%) | 0.47 |
| Yes | 13 (57%) | 9 (60%) | ||
| Smoker | No | 10 (43%) | 12 (75%) | 0.06 |
| Yes | 13 (57%) | 4 (25%) | ||
| Previous Cardiac Surgery | No | 18 (78%) | 13 (81%) | 0.82 |
| Yes | 5 (22%) | 3 (19%) | ||
| Ischemic Heart Disease | No | 21 (91%) | 15 (94%) | 0.8 |
| Yes | 2 (9%) | 1 (6%) | ||
| Moderate-severe mitral valve disease | No | 23 (100%) | 15 (94%) | 0.23 |
| Yes | 0 (0%) | 1 (6%) | ||
| LVEF <55% | No | 2 (9%) | 2 (12%) | 0.7 |
| Yes | 21 (91%) | 14 (88%) | ||
| Beta-blockers | No | 9 (41%) | 7 (47%) | 0.91 |
| Yes | 13 (59%) | 8 (53%) | ||
| AAD | No | 13 (57%) | 5 (31%) | 0.12 |
| Yes | 10 (43%) | 11 (69%) | ||
| AAD group 1C | No | 19 (83%) | 13 (81%) | 0.91 |
| Yes | 4 (17%) | 3 (19%) | ||
| Amiodarone | No | 18 (78%) | 12 (75%) | 0.81 |
| Yes | 5 (22%) | 4 (25%) | ||
| OAC | No | 10 (43%) | 4 (25%) | 0.24 |
| Yes | 13 (57%) | 12 (75%) | ||
| NOAC | No | 16 (70%) | 13 (81%) | 0.41 |
| Yes | 7 (30%) | 3 (19%) | ||
| VKA | No | 17 (74%) | 7 (44%) | 0.06 |
| Yes | 6 (26%) | 9 (56%) | ||
| Type of AF | Paroxysmal | 12 (52%) | 9 (56%) | 0.8 |
| Persistent | 11 (48%) | 7 (44%) | ||
| LA diameter >45mm | No | 13 (57%) | 10 (63%) | 0.71 |
| Yes | 10 (43%) | 6 (37%) | ||
| LA VOLi >40ml/m2 | No | 20 (87%) | 9 (56%) | 0.03 |
| Yes | 3 (13%) | 7 (44%) | ||
| CHADS2 | 0.91 (0-1.8) | 1 (0-1.5) | 0.66 | |
| CHA2DS2-VASc | 1.26 (0-2.26) | 1.7 (0-2.5) | 0.36 | |
| Sphericity Index | 1.18 (0.85-1.51) | 1.26 (0.82-1.7) | 0.34 | |
| AF: Atrial Fibrillation; HTA: Hypertension; AAD: Anti-Arrhythmic Drugs; LA VOLi: Left Atrial Volume Index; NOAC: Non-Vitamin K Antagonist Oral Anticoagulant; OAC: oral anticoagulation, VKA: Vitamin K Antagonist | ||||
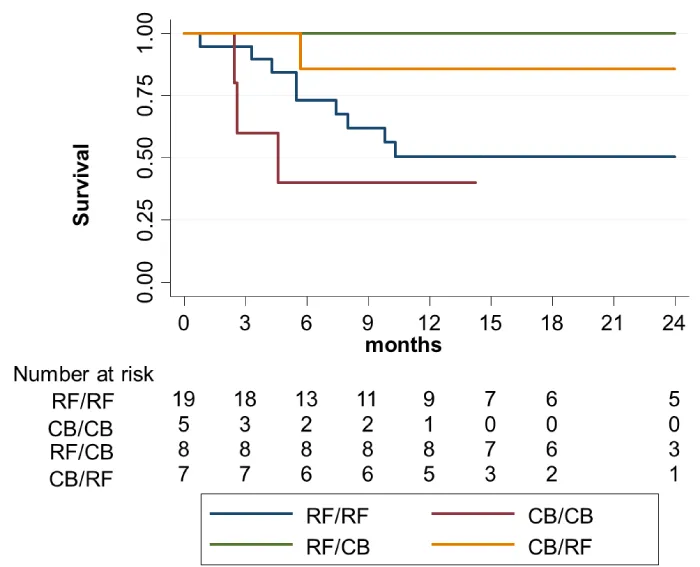
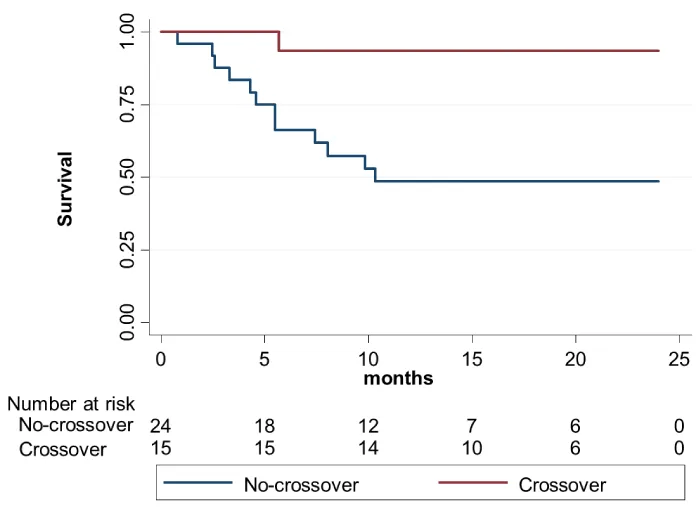
AF recurrence-free survival during follow up for the four groups is showed in figure 1, pointing to a clear difference in AF recurrence rates after second ablation procedure when two different energies were used, independently of its order, even despite the small number of patients in each group. When the sample was grouped in crossover and no-crossover groups, survival analysis figure 2 showed that AF recurrence was significantly lower in the group of interest (crossover group) after a median follow-up of 18.5 (7.4-29.9) months (Log rank 8.540,p = 0.003).
Figure 1: Kaplan-Meier AF recurrence-free survival of the four groups separately.
Figure 2: Kaplan-Meier AF recurrence-free survival for the crossover (CB-RF and RF-CB) vs. no-crossover groups (RF-RF and CB-CB).
PVI after the second procedure was 100% in all patients in both groups. No deaths or hospitalizations occurred during follow up (data censored at 24h moths). At 1 year, arrhythmia free-survival was significantly higher in the crossover group compared to control group [93,3% vs. 47,8%; HR 0.19 (0.06-0.66);p = 0,009].
Nowadays, point by point RF and CB ablation are the two most studied and established techniques to perform PVI. Although no differences in efficacy and safety were found after first procedure 4–7, there are important differences between both techniques [8-10]. RF requires only limited use of fluoroscopy, because catheter guidance is achieved with the use of an electroanatomical mapping system, but the approach demands extensive training [11-16]. CB for atrial fibrillation requires more extensive fluoroscopic guidance to position the balloon catheter at the pulmonary veins ostia and check for pulmonary vein occlusion by using intravenous contrast. The cryoballoon was developed to create a circular lesion around each pulmonary vein ostium in a simple manner, simplifying the technique and allowing its generalization [16]. Most recent evidence and randomized trials using lines, complex and fractionated electrograms ablation and even rotor ablation have been discouraging [17].
Despite the evolution of technology, around thirty percent of patient have a recurrence of AF after PVI independently of the technique used and even more when dealing with long-standing persistent AF [18,19]. When facing an AF recurrence after PVI is not clear what to do during the second procedure, and even in the most recent guidelines [3,4] there is no clear recommendation beyond identifying the reconnected veins or how to re-isolate them.
Recent published evidence comparing the best potential technique for both approaches (Cryoballoon Antral Pulmonary Vein Isolation vs. Force-Sensing Radiofrequency Catheter Ablation for Pulmonary Vein and Posterior Left Atrial Isolation) showed no differences in efficacy between the two approaches after the first ablation procedure in patients with persistent AF. Nevertheless, and contrary to our approach, in this study the second ablation procedure in case of recurrence was performed using RF in the whole population, so making our approach for second procedure ablation unique and never reported so far [7]. Furthermore, given the lack of evidence in the literature regarding the best approach for a second AF ablation procedure, our approach is the first specifically focusing on it.
Main findings of our study were: 1. PVI can be achieved in 100% of the patients after a second ablation procedure no matter the energy used. 2. AF-free survival is significantly improved by means of crossing the ablation energy used for the first procedure (from point by point RF to CB and the other way around). The clear net separation in AF recurrence-free survival analysis from very beginning after the ablation procedure points to a better long-term duration of PVI when two different energies are combined in the same patient. We did not find any clinical or anatomical characteristic that could explain the results, but we can speculate that a potential synergistic effect of combining two ablation energies, with an overcoming effect of each one limitations for certain areas of pulmonary veins antra could explain the results. In addition to this, not only the different energies but the different ways of energy delivery (single shot balloon ablation vs. sequential point-by-point catheter tip ablation) may also have a complementary effect explaining the results, but these hypothesis remain to be proven.
It is important to emphasize the potential synergistic effect of cryo energy and radiofrequency, due to differences in the type of injury that they generate [9] (type of necrosis, depth and location of the lesion), potentially overcoming anatomical barriers for transmural ablation of each energy when both are combined in the same patient; while RF leads to cellular necrosis by tissue heating, CB uses cryogenic energy which leads to necrosis by freezing with minimal endocardial surface disruption and less thrombogenic risk [20]. Modification of the ganglionated plexi by the ablation procedure is one of the accepted mechanisms behind AF ablation success. If the crossover approach, combining two different ablation energies in two different procedures, creates some kind of synergistic effect over the ganglionated plexi and helps to improve the results is an appealing theory, but remains to be proven.
Neither ablation index for RF nor time to effect for CB ablation were the standard approach for the first patients included in our analysis as they are nowadays, and were not used to guide the ablation procedure for the first cases. So we decided not to include those data, even retrospectively reanalyzed. This could generate a potential bias if any energy was applied more effectively than the other. Anyway, this could explain potential better results with CB or with RF as a whole, when the same energy was used for the first and the second procedure. But if any energy was delivered more effectively than the other, this could not explain the better results encountered in the crossover group compared to the no-crossover group.
Left atrial volume index (LA VOLi), but not diameter, was globally higher in the crossover group (44% with LA VOLi > 40 ml/m2). Its influence, if any, gives even more consistency to the results encountered, given the worse predicted result of AF ablation in patients with left atrial dilatation. Contrary to what we could expect in patients with left atrial dilatation (mostly in the crossover group), the 1-year rate free from AF was much higher in this group than the no-crossover group (93,3% vs. 47,8%). We tried unsuccessfully to find anatomical characteristics associated with our results, such as atrial size, number of veins or their conformation (common trunk, presence of supernumerary veins), sphericity index (both independently for each vein or globally promediated) and none of them showed any correlation with the results observed. However, although we could not find differences in sphericity index, we should remark that the sample size is small. More studies are necessary to corroborate our results and to search if sphericity index or other anatomic factors (like ridge thickness or vein disposition) may explain the results and potentially predict which technique best suits which patient.
The main hypothesis of our study was to try to proof than, instead of additional ablation on top of complete PVI, assure PVI by changing the ablation energy for the second procedure could improve the results, as was finally shown. In summary, according to our results, although both ablation techniques and energies are considered equivalent as an initial approach, and also the acute results are similar for a second ablation procedure, when facing an AF recurrence it would be desirable to change the ablation technique first used (CB or point-by-point RF) to improve the AF-free survival during follow-up.
This is a retrospective non-randomized non-blinded analysis, so there are many potential confusing factors not controlled by randomization. 22 baseline clinical, pharmacological and anatomical characteristics were equally distributed, apart from left atrial volume, between groups, but many others could potentially influence the results.
The main limitation is the small sample size for each group (crossover vs. no-crossover), but the impressive differences encountered warrant further investigation with a higher volume of patients. Another limitation is the unicenter nature of the study, so the results should be replicated in a multicenter trial in order to be confirmed. In any case, the technique used for point-by-point RF ablation and CB ablation followed the standardized approach commonly and widely adopted. The CB dose used (4 min first application and 3 min bonus afterwards) is nowadays under intense investigation, and the results should be confirmed in case any other dose is used.
Another potential limitation is the lack of intense follow-up by means of transtelephonic monitoring, long duration ECG-monitoring or implantable loop recorders, but due to the fact that both groups (crossover and no-crossover) used the same follow-up protocol, is highly unlikely that closer follow-up would have changed the differences encountered (absolute number would be different, but the relative difference would remain).
When facing an AF recurrence after PVI is not clear what strategy is best for the second ablation procedure, and even in the most recent guidelines there is no clear recommendation apart from re-isolating the veins. According to our data, the best ablation approach after AF recurrence should be to cross the ablation energy and technique, irrespective of it was CB or point-by-point RF at the initial procedure. This crossover approach is associated with the same acute success, but it has significant less long-term AF recurrence and time to recurrence. Potential synergistic effect of radiofrequency and cryoballoon ablation should be explored further in future studies.
- Stewart S, Hart CL, Hole DJ, McMurray JJ. A Population-Based Study of the Long-Term Risks Associated with Atrial Fibrillation: 20-Year Follow-up of the Renfrew/Paisley Study. Am J Med. 2002: 113: 359–364. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12401529
- Lafuente-Lafuente C1, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for Maintaining Sinus Rhythm after Cardioversion of Atrial Fibrillation. Cochrane Database Syst Rev. 2015; CD005049. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25820938
- Kirchhof P Benussi S1, Kotecha D, Ahlsson A, Atar D, et al. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with EACTS. Eur Heart J. 2016; 50:, e1-e88. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27663299
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; 64: e1-76. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24685669
- Squara F, Zhao A, Marijon E, Latcu DG4 Providencia R, et al. Comparison between Radiofrequency with Contact Force-Sensing and Second-Generation Cryoballoon for Paroxysmal Atrial Fibrillation Catheter Ablation: A Multicentre European Evaluation. Europace. 2015; 17: 718–724. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25840289
- Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2016; 374: 2235–2245. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27042964
- Yokokawa M, Chugh A, Latchamsetty R, Ghanbari H, Crawford T, et al. Cryoballoon Antral Pulmonary Vein Isolation vs. Force-Sensing Radiofrequency Catheter Ablation for Pulmonary Vein and Posterior Left Atrial Isolation in Patients with Persistent Atrial Fibrillation. Heart Rhythm. 2018; 15: 1835-1841. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30509365
- Schmidt M, Dorwarth U, Andresen D, Brachmann J, Kuck KH, et al. Cryoballoon versus RF Ablation in Paroxysmal Atrial Fibrillation: Results from the German Ablation Registry. J Cardiovasc Electrophysiol. 2014; 25: 1–7. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24134539
- Fitzgerald DM. Catheter Ablation of Atrial Fibrillation: To Freeze, or Not to Freeze, That Is the Question. J Cardiovasc Electrophysiol. 2014; 25: 8–10. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24112780
- Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, et al. Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients With Paroxysmal Atrial Fibrillation: The Prospective, Randomized, Controlled, Noninferiority FreezeAF Study. Circulation. 2015; 132: 1311–1319. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26283655
- Wasserlauf J, Pelchovitz DJ, Rhyner J, Verma N, Bohn M, et al. Cryoballoon versus Radiofrequency Catheter Ablation for Paroxysmal Atrial Fibrillation. Pacing Clin Electrophysiol. 2015; 38: 483–489. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25627795
- Koch L, Haeusler KG, Herm J, Safak E, Fischer R, et al. Mesh Ablator vs. Cryoballoon Pulmonary Vein Ablation of Symptomatic Paroxysmal Atrial Fibrillation: Results of the MACPAF Study. Europace. 2012; 14: 1441–1449. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22523379
- Kuck KH, Fürnkranz A, Chun KR, Metzner A, Ouyang F, et al. Cryoballoon or Radiofrequency Ablation for Symptomatic Paroxysmal Atrial Fibrillation: Reintervention, Rehospitalization, and Quality-of-Life Outcomes in the FIRE and ICE Trial. Eur Heart J. 2016; 37: 2858–2865. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27381589
- Natale A, Nielsen JC. To Burn or to Freeze: A Burning Question yet to Be Resolved. Eur Heart J. 2016; 37: 2866–2868. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27593103
- Mugnai G, Sieira J, Ciconte G, Hervas MS, Irfan G, et al. One Year Incidence of Atrial Septal Defect after PV Isolation: A Comparison between Conventional Radiofrequency and Cryoballoon Ablation. Pacing Clin Electrophysiol. 2015; 38: 1049–1057. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25974316
- Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, et al. Results from a Multicentre Comparison of Cryoballoon vs. Radiofrequency Ablation for Paroxysmal Atrial Fibrillation: Is Cryoablation More Reproducible? Europace. 2017; 19: 48–57. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27267554
- Miyazaki S1, Hachiya H1, Nakamura H1, Taniguchi H1, Takagi T, et al. Pulmonary Vein Isolation Using a Second-Generation Cryoballoon in Patients With Paroxysmal Atrial Fibrillation: One-Year Outcome Using a Single Big-Balloon 3-Minute Freeze Technique. J. Cardiovasc. Electrophysiol. 2016; 27: 1375–1380. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27534931
- Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N Engl J Med. 2015; 372: 1812–1822. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25946280
- Providência R, Lambiase PD, Srinivasan N, Ganesh Babu G, Bronis K, et al. Is There Still a Role for Complex Fractionated Atrial Electrogram Ablation in Addition to Pulmonary Vein Isolation in Patients with Paroxysmal and Persistent Atrial Fibrillation? Circ Arrhythmia Electrophysiol. 2015; 8: 1017–1029. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26082515
- Siu CW, Tse HF. Thromboembolic Risk of the Hot- and Cold-Catheter Ablation for Atrial Fibrillation. Hear Rhythm. 2012; 9: 197–198. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21978961