More Information
Submitted: 26 February 2020 | Approved: 18 May 2020 | Published: 19 May 2020
How to cite this article: Seghda TAA, Boro T, Bambara JE, Cisse K, Lopez AM, et al. Prognosis of peripartum cardiomyopathy in sub-Saharan Africa (Burkina Faso South-West PPCM register). J Cardiol Cardiovasc Med. 2020; 5: 109-113.
DOI: 10.29328/journal.jccm.1001096
ORCiD: orcid.org/0000-0003-3840-9322
Copyright License: © 2020 Seghda TAA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Peripartum cardiomyopathy; Prognosis; Delay diagnosis
Prognosis of peripartum cardiomyopathy in sub-Saharan Africa (Burkina Faso South-West PPCM register)
Taryètba André Arthur Seghda1*, Théodore Boro2, Jean Eudes Bambara1, Kadari Cisse3, Andrés Miguel Lopez4, André K Samadoulougou4 and Patrice Zabsonre5
1Cardiology department, Regional Hospital of Gaoua, Burkina Faso
2Saint Camille Hospital of Ouagadougou, Burkina Faso
3Institute for Health Sciences Research, Ouagadougou, Burkina Faso
4University Hospital of Bogodogo, Burkina Faso
5University Hospital of Yalgado Ouédraogo, Ouagadougou, Burkina Faso
*Address for Correspondence: Taryètba André Arthur Seghda, CHU de Bogodogo, Ouagadougou, Burkina Faso, Tel: +226 71 61 69 94; +226 66 04 48 48; Email: [email protected]
Peripartum cardiomyopathy is one of the curable cardiomyopathy. It’s a severe and frequent disease arising among women of childbearing age. Its evolution in the long-term among some patients leads to chronic heart failure. Our study aims to determine from a prospective cohort, the factors associated with the non-recovery of myocardial function upon 12 months of diagnosis. Sociodemographic, clinical and echocardiographic data were collected at the time of diagnosis and then in months 3, 6 and 12. The outcome was the non-recovery of myocardial function at one year, defined by a left ventricular ejection fraction (LVEF) below 50%. 60 patients were analyzed after 12 months of follow-up. Mortality was about 13.3% and recovery rate of myocardial function reached 42.3%. After logistic regression, delay diagnosis and observance were the factors related to non- recovery of myocardial function.
Peripartum cardiomyopathy (PPCM) can be defined as the occurrence of congestive heart failure in the peripartum in the absence of any other etiology. It caused a left ventricular (LV) systolic dysfunction with an ejection fraction (LVEF) below 45% and most often, a left ventricular dilation above 2.7 cm/m2 [1]. This is a major public health issue since its occurrence can be a turning point in the life of young primiparous women whose obstetric future will be compromised due to severe ventricular dysfunction, therefore forbidding further pregnancies [2]. Sometimes, this issue also concerns grand ultiparous women whose life expectancy can be highly reduced by premature death. Very few studies in Africa were performed on the survival of these patients. From a classic perspective, this is one of the causes of curable left ventricular systolic dysfunction [3-6]. The non-recovery reasons are not known but according to some studies, the chances to get recovered are strongly compromised in the absence of a normalized LV systolic function after 3 months. Our study rationale is the necessity to reduce morbi-mortality in peripartum cardiomyopathy by analyzing the prognosis upon one year of the diagnosis.
This is a prospective and analytical cohort in which patients were recruited from three centers in the south-west region of Burkina Faso. Inclusion started on March 1st, 2016. The purpose of this study was to determine the prognosis and then, the factors associated with the non-recovery of LV systolic function upon 12 month of the diagnosis. Patients suffering from heart failure symptoms along with a LVEF < 45% on echocardiography, from the last quarter of pregnancy to the first five months of postpartum, were consecutively received and included in the study. Non-inclusion criteria were the presence of severe chronic anemia (hemoglobin less than 8g/dl), thyroid dysfunction or pre-existing cardiac or chronic renal diseases. Data were systematically collected at the very first contact and then upon three, six and twelve months. Socio-demographic data including age, socioeconomic level and number of parity were the study variables. As for clinical data, these included clinical signs, diagnosis delay, complications and treatment provided. Echographic data LV diameter and ejection fraction measured by the Simpson method. Biological data were hemoglobin and serum creatinine. Non-recovery of systolic function at one year was defined by LV systolic function < 50%. Observance was deemed good if the treatment was not interrupted more than one week. Morbi- mortality rates were estimated by death and rehospitalization. We conducted a bivariate comparative analysis of the two groups of patients namely, those with a one-year LVEF ≥ 50% and the others with LVEF < 50%. We assumed that our data were complying with a normal law. The Chisquare test was used with a significance threshold value p ≤ 0.05. The correlation between ventricular diameters, ejection fraction, and time limit for treatment provision was assessed through the visual appreciation of point clouds tendency approximated by a straight line and correlation coefficient r. The correlation will be deemed moderate to good and strong to excellent, respectively for coefficients r between 0.5-0.74 and 0.75-1. Factors associated with non-recovery of myocardial function were determined based on multivariate logistic regression.
From enrollment to follow-up period going from March 1st, 2016 to June 31st, 2018, 66 female patients suffered from a CMPP among which 6 were out of reach. The average duration of follow-up was 318.2 days ± 14.2; IC [289.6; 346.7]. Eight deaths were reported, corresponding to a mortality rate of 13.3%. At the end of 12 months of follow-up, 22 patients had their LVEF higher or equal to 50%, corresponding to a recovery rate of 42.3%.
Sociodemographic features and medical treatment of patients
Patients’ mean age was 26.6 years± 7.39, CI to 95% [24.7; 28.5] and all of them had a low socio-economic level. The average number of pregnancies per woman was 3.4 ± 2.4 with extremes estimated at 1 and 14. Primiparous and grand multiparous women (number of parity ≥ 4) respectively represented 18.3% and 35%. As for therapeutic drug perspective, patients were treated with diuretics (100%), angiotensin-converting enzyme inhibitors or angiotensin receptors blockers (100%), spironolactone (n = 50, 96.1%), beta blockers (n = 24). 46.1%), bromocriptine (n = 6, 11.5%) and vitamin K antagonists (n = 2, 3.8%).
Clinical and echocardiographic variables predicting a non-recovery of myocardial function at 12 months
In univariate analysis, advanced age, diagnosis delay, and observance (Table 1) were the clinical predictors of non-recovery of myocardial function upon one year.
| Table 1: Clinical preditors of non-recovery of myocardial function at one year. | ||||
| Variables | General population (n = 52) |
LVEF < 50% (n = 30) | LVEF ≥ 50% (n = 22) | p |
| Mean age (years) | 27.2 ± 7.7 | 29.5 ± 8.1 | 24 ± 5.9 | 0.005 |
| Prepartum diagnosis | 2 (3.85%) | 2 (6.67%) | 0 (0%) | 0.217 |
| Mean diagnosis delay (days) | 53.5 ± 59.1 (37.1 ; 70) | 79 ± 64.4 (54.9 ; 103) | 18.9 ± 24 (8.2 ; 29.59) | 0.0001 |
| Early diagnosis | 30 (57.6%) | 12 (40%) | 18 (81.8%) | |
| Complication at admission | 17 (32.7%) | 13 (43.3%) | 4 (18.2%) | 0.003 |
| Poor adherence | 27 (51.9%) | 20 (66.7%) | 7 (31.8%) | 0.056 |
| 0.013 | ||||
Still regarding the univariate analysis, LV diameter especially when it was much dilated and altered ejection fraction were echocardiographic predictors (Table 2).
| Table 2: Echocardiographic predictors of non-recovery of myocardial function at one year. | ||||
| Variables | Total group (52) | LVEF < 50% (n = 30) | LVEF ≥50% (n = 22) | p |
| Echocardiographic data | ||||
| LVEDD0 | 62.1 ± 5.7(60.5 ; 63.7) | 64.6 ± 5.3(62.6 ; 66.6) | 58.8 ± 4.4(56.8 ; 60.7) | p < 0.001 |
| LVEDD 3 | 58.3 ± 6.3(56.5 ; 60.1) | 60.7 ± 6.24(58.3 ; 63.1) | 55.1 ± 4.8(52.9 ; 57.3) | 0.0006 |
| LVEDD 6 | 56.6 ± 7.2(54.6 ; 58.7) | 59.8 ± 6.9(57.2 ; 62.3) | 52.4 ± 5.2(50.1 ; 54.7) | p < 0.001 |
| LVEF0 | 28.6 ± 8.04(26.3 ; 30.8) | 26.7 ± 8.5(23.5 ; 29.9) | 31.1 ± 6.6(28.2 ; 34.1) | 0.023 |
| LVEF 3 | 39.2 ± 11.5 (36 ; 42.5) | 32.9 ± 9.5(29.3 ; 36.5) | 47.6 ± 8.2 (44 ; 51.3) | p < 0.001 |
| LVEF 6 | 43.4 ± 13 (39.8 ; 47) | 35.6 ± 10.2(31.8 ; 39.5) | 54 ± 7.8 (50.5 ; 57.4) | p < 0.001 |
| LVEDD: left ventricle end diastolic diameter, (0: at admission; 3: at three months; 6: at six months). | ||||
| LVEF: left ventricle ejection fraction, (0: at admission; 3: at three months; 6 at six months). | ||||
Correlation between diagnosis delay and echocardiographic predictors of non-recovery of myocardial function
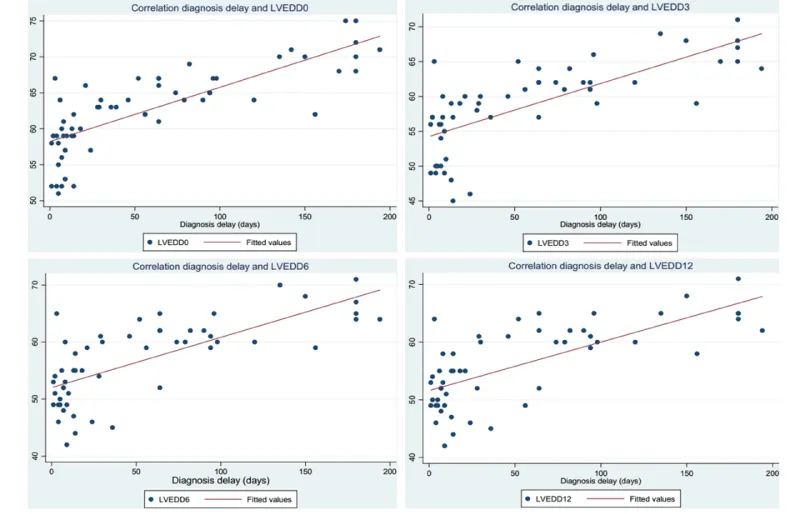
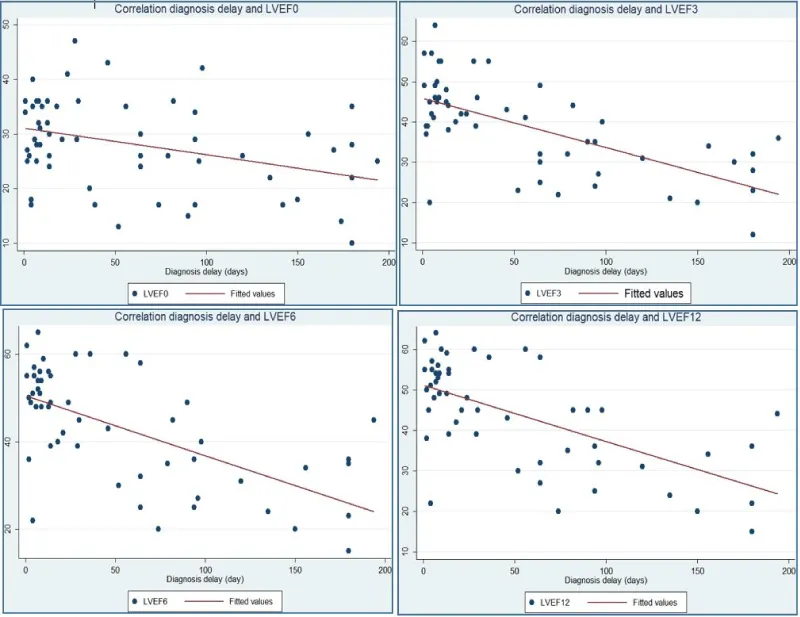
LV end-diastolic diameter at the time of diagnosis (LVEDD0) is the most positively correlated with diagnosis delay (correlation coefficient 0.79). LV ejection fraction at the time of diagnosis (LVEF0) is the least negatively correlated with diagnosis delay (correlation coefficient -0.36).
Graph 1 analyze correlation between LVEDD, measured at admission, at three, six and twelve months and the diagnosis timelines. Graph 2 analyze correlation between LVEF, measured at admission, at three, six and twelve months and the diagnosis delay.
Graph 1:
Graph 2:
Factors associated with non-recovery of myocardial function at 12 months.
In multivariate analysis, diagnosis delay and observance were factors associated to non-recovery of myocardial function (Table 3).
| Table 3: Variables associated to non-recovery of myocardial function at one year. | ||||
| Variables | Coefficient | IC 95% | p | |
| Diagnosis timelines | -0.002 | -0.0045 ; -.000 | 0.042 | |
| Therapeutic compliance | -0.36 | -0.57 ; -0.15 | 0.001 | |
| LVEDD0 | -0.013 | -0.04 ; 0.016 | 0.36 | |
| LVEF0 | 0.0019 | -0.010 ; 0.014 | 0.76 | |
| Complication at admission | -0.24 | -0.51 ; 0.02 | 0.075 | |
| LVEDD0: left ventricle end diastolic diameter at admission. | ||||
| LVEF0: left ventricle ejection fraction at admission. | ||||
PPCM is a potentially severe disease. In absence of a normal LVEF, female patients will be classified in the stage mWHO III or mWHO IV of the modified maternal risk classification of WHO. With these stages, such female patients have a cardiovascular occurrence risk in further pregnancies raising from 27% to 100% including maternal-fetal death [2]. In 1957, Meadows noted a recurrence rate of 50% with long-term irreversible inflammatory myocardial fibrosis [7].
The mortality at 12 months after diagnosis in our series was 13.3% and the recovery rate of LV myocardial function was 42.3%. Like many authors, we found a moderate to good correlation between diagnostic timelines and LVEF at 3; 6 and 12 months on one hand, and with left ventricular diameters on the other [3,5,8,9].
The later the diagnosis, the more dilated and severe will be the left ventricle and the more impaired will be the LVEF. As for univariate analysis, advanced age, delayed diagnosis, non-compliance to treatment, significant LV dilation, and collapsed ejection fraction were predictors of non-recovery of myocardial function at one year. However, we found in multivariate analysis after logistic regression that only delayed diagnosis and non-compliance to treatment were the factors statistically associated with non-recovery of myocardial function one year after the diagnosis. Our small-sized sample is the shortcoming of our results, which did not enable us to include a larger number of explanatory variables.
The delayed diagnosis favors the onset of myocardial fibrosis which impedes on the recovery chances. The diagnosis average timeline was 79 days, approximately 3 months, for the group of female patients whose LVEF upon one year is not ≥ 50%. The unfavorable socio-economic conditions are the causes of delayed consultation. Beyond this period, the recovery chances would be very low [10].
Recovery rates above 72% were observed in the SCPI study in North America, reaching 86% in patients whose initial LVEF ≥ 30% [8]. It is quite difficult to compare these results to ours because of the early diagnosis and the differences in therapeutics. Bromocriptine was used in very few cases in our study (11.5%). This molecule has shown very satisfactory results in terms of myocardial function recovery in many series [3,6,11,12]. The reasons for not prescribing the bromocriptine in our study were related to poverty on one hand and on the other hand to the delayed diagnosis since its prescription would not be relevant at this
stage. In our study a low number of PPCM is treated with beta blocker (46%), this data can explain the elevate mortality and non-recovery of myocardial function.
Some other authors have found factors related to poor prognosis in the long term.
Such factors include the existence of the following at the diagnosis time: right ventricular dysfunction, LVEDD above 60 mm and ejection fraction below 30% [13]. Gestational high blood pressure and preeclampsia are more likely associated with a high recovery rate [14,15].
PPCM is a severe disease among women of childbearing age. Although its initial presentation at diagnosis and its short-term evolution are unpredictable, clinical predictors of non-recovered myocardial function exist. Early treatment with heart failure drugs is the key solution to healing.
- European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM), Regitz-Zagrosek V, Blomstrom Lundqvist C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32: 3147‑3197. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21873418
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, et al. ESC Scientific Document Group. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018; 39: 3165‑3241. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30165544
- Yaméogo NV, Kagambèga LJ, Seghda TAA, Owona A, et al. Bromocriptine in Management of Peripartum Cardiomyopathy: A Randomized Study on 96 Women in Burkina Faso. J Cardiol Clin Res. 2017; 5: 1098.
- Kane Ad, Mbaye M, Ndiaye MB, Diao M, Moreira PM et al.. Évolution et complications thromboemboliques de la myocardiopathie idiopathique du péripartum au CHU de Dakar : étude prospective à propos de 33 cas. J Gynécologie Obstétrique Biol Reprod. 2010; 39: 484‑489.
- Pio M, Afassinou Y, Baragou S, Akue EG, Péssinaba S, et al. Particularités de la cardiomyopathie du péripartum en Afrique: le cas du Togo sur une étude prospective de 41 cas au Centre Hospitalier et Universitaire Sylvanus Olympio de Lomé. PAMJ. 2014; 17: PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4189861/
- Habedank D, Kühnle Y, Elgeti T, Dudenhausen JW, Haverkamp W, et al. Recovery from peripartum cardiomyopathy after treatment with bromocriptine. Eur Heart J. 2008; 10:1149‑1151. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18926768
- Meadows WR. Idiopathic Myocardial Failure in the Last Trimester of Pregnancy and the Puerperium. Circulation.1957; 15: 903‑914. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/13437416
- McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, et al. IPAC Investigators. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015; 66: 905‑914. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26293760
- Evolution et facteurs pronostiques des cardiomyopathies du péripartum à Lomé. Cardiol Trop. 2013; 146.
- Vanzetto G. Cardiomyopathie du péripartum. Le Praticien en Anesthésie Réanimation. 2013; 17: 180‑186.
- Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 2017; 38: 2671‑2679. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28934837
- Elkayam U, Goland S. Bromocriptine for the treatment of peripartum cardiomyopathy. Circulation. 2010; 121: 1463‑1464. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28934837
- Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol. 1997; 176: 182‑188. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9024111
- Lindley KJ, Verma AK, Blauwet LA. Peripartum Cardiomyopathy: Progress in Understanding the Etiology, Management, and Prognosis. Heart Fail Clin. 2019; 1529‑1539. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30449378
- Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, et al. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol. 2012; 154: 27‑31. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20863583