More Information
Submitted: October 11, 2020 | Approved: December 15, 2020 | Published: December 16, 2020
How to cite this article: Vanderheyden M, Verstreken S, Goethals M. Post-extrasystolic potentiation differentiates “true” from “pseudo” Low-flow, Low-gradient aortic stenosis. J Cardiol Cardiovasc Med. 2020; 5: 172-173.
DOI: 10.29328/journal.jccm.1001105
Copyright License: © 2020 Vanderheyden M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Post-extrasystolic potentiation differentiates “true” from “pseudo” Low-flow, Low-gradient aortic stenosis
Marc Vanderheyden*, Sofie Verstreken and Marc Goethals
Cardiovascular Center, Onze Lieve Vrouwe Ziekenhuis, Aalst, Belgium
*Address for Correspondence: Dr. Marc Vanderheyden, Cardiovascular Center, Onze Lieve Vrouwe Ziekenhuis, Moorselbaan 164, B-9300, Aalst, Belgium, Tel: +32 53 724 439; Fax: +32 53 724 768; Email: [email protected]
Post-extrasystolic potentiation (PESP) is a marker of contractile reserve and refers to the augmentation of left ventricular contractility due to preload recruitment and rise in intracellular calcium following a premature beat. In this case report we show that PESP might be a safe and helpful aid to evaluate low flow, low gradient aortic stenosis and contractile reserve in the cathlab, thereby reducing the potential risk of complications associated with intravenous dobutamine evaluation and reducing unnecessary testing.
In patients with left ventricular (LV) dysfunction and low flow, low gradient aortic stenosis (LF-LGAS), patients with “true-severe AS” (severe AS with secondary LV dysfunction) can be distinguished from those with “pseudo-severe AS” (moderate AS with associated LV dysfunction) by dobutamine stress echocardiography (DSE) [1].
Post-extrasystolic potentiation (PESP) is a marker of contractile reserve and refers to the augmentation of left ventricular contractility due to preload recruitment and rise in intracellular calcium following a premature beat [2]. Whereas normal subjects exhibit no or only a slight change in LV systolic pressure without any change in transaortic gradient [2], a PESP associated increase in LV systolic pressure together with a rise in transaortic pressure gradient has been reported in AS pts [3]. In this regard PESP can help to distinguish between pseudo-severe and severe AS in LF-LGAS [4,5] .
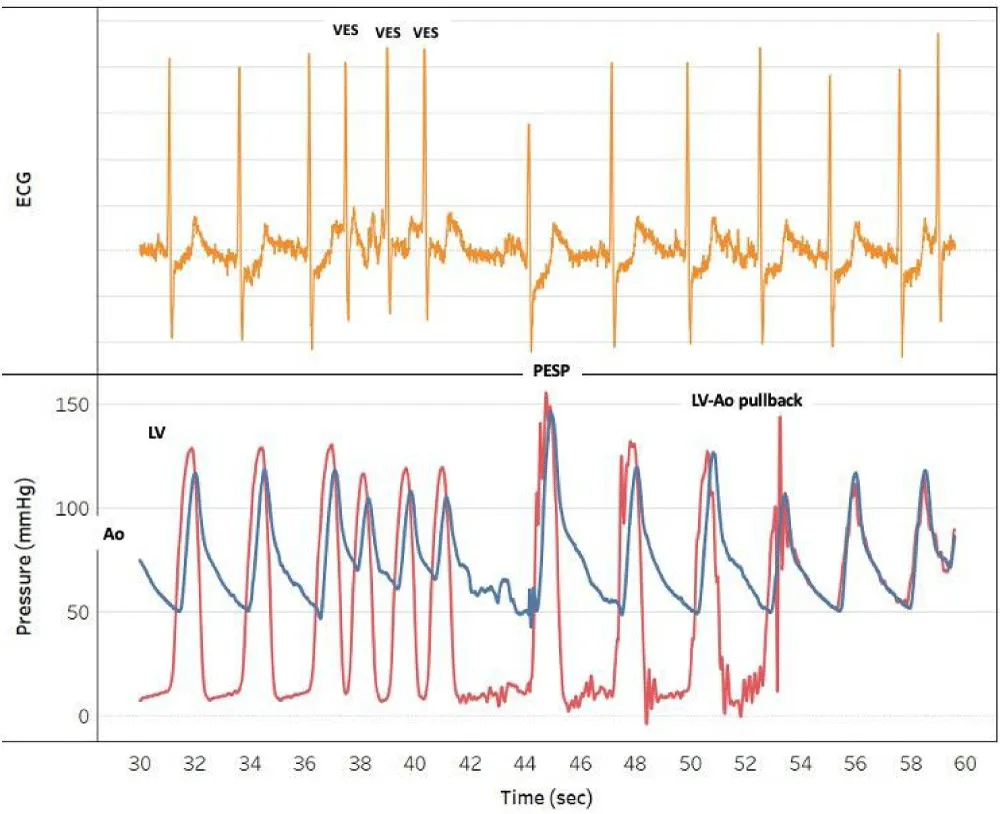
We report on a 89-year old patient, admitted with acute heart failure due to LF-LGAS. Invasive hemodynamics demonstrated an AS with mean gradient of 20 mm Hg and valve area of 0,9 cm2 at rest, in line with the echo findings. The LV was severely dilated (EDVI: 125 cml/m2), and EF was depressed (EF: 11%). However, PESP demonstrated contractile reserve evidenced by a significant rise in LV pressure together with a disappearance of the transaortic gradient (Figure 1).
Figure 1: Post-extrasystolic potentiation(PESP) demonstrates contractile reserve and unmasks “pseudo-severe” AS; LV indicates Left Ventricle, Ao: Aorta
These observations can only be explained by a better opening of the aortic valve due to a PESP-mediated increase in stroke volume, indicating that LV dysfunction not AS is responsible for the observed transaortic gradient. A diagnosis of pseudo-severe AS was made and medical therapy was initiated.
This case report demonstrates that PESP might be a safe and helpful aid to evaluate LF-LGAS and contractile reserve in the cathlab, thereby reducing the potential risk of complications associated with intravenous dobutamine evaluation and reducing unnecessary testing [5].
Consent
The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017; 38: 2739–2791. PubMed: https://pubmed.ncbi.nlm.nih.gov/28886619/
- Wisenbaugh T, Nissen S, DeMaria A. Mechanics of postextrasystolic potentiation in normal subjects and patients with valvular heart disease. Circulation. 1986; 74: 10–20. PubMed: https://pubmed.ncbi.nlm.nih.gov/2423268/
- Domingo E, Angel J, Alvarez A, Anivarro I, Soler‐Soler J. Postextrasystolic potentiation of systolic gradient in valvular aortic stenosis: Clinical usefulness and analysis of hemodynamic factors. Catheter Cardio Diag. 1987; 13: 381–390. PubMed: https://pubmed.ncbi.nlm.nih.gov/2446771/
- Wiley BM, Pollack A, Vaidya AS, Agarwal SK, Sengupta PP, et al. Post-Extrasystolic Transaortic Valve Gradients Differentiate “Pseudo” and “True” Low-Flow, Low-Gradient Severe AS During Dobutamine Stress Echocardiography. Jacc Cardiovasc Imaging. 2017; 10: 1199–1200. PubMed: https://pubmed.ncbi.nlm.nih.gov/28109925/
- Bhave NM, Patel AR, Shah AP, Lang RM. Postextrasystolic Potentiation in Low‐Gradient, Severe Aortic Stenosis: A Poor Man’s Stress Echo? Echocardiogr. 2013; 30: E148–151. PubMed: https://pubmed.ncbi.nlm.nih.gov/23551839/