More Information
Submitted: March 23, 2022 | Approved: March 29, 2022 | Published: March 30, 2022
How to cite this article: Puppo AM, Caro MF, Sastre SM, Gómez FT, Sánchez JML. Fibromuscular dysplasia and aortic dissection. J Cardiol Cardiovasc Med. 2022; 7: 023-025.
DOI: 10.29328/journal.jccm.1001127
Copyright License: © 2022 Puppo AM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Fibromuscular dysplasia and aortic dissection
Antonio M Puppo1,3*, Manuel Fernández Caro1, Sara Martín Sastre1, Francisco T Gómez2 and Jose Mariá López Sánchez1
1Critical Care Surgery Unit, Virgen del Rocío University Hospital, Sevilla, Spain
2Vascular Surgery Department, Seville University, Spain
3Medicine School, Seville University, Spain
*Address for Correspondence: Antonio M Puppo, Critical Care Surgery Unit, Virgen del Rocío University Hospital, Sevilla, Spain, Email: [email protected]
Fibromuscular dysplasia is a rare, non-atherosclerotic, non-inflammatory vascular disease that typically affects women between the ages of 20 and 60 years.
Although any artery can be affected fibromuscular dysplasia most commonly affects the renal and carotid arteries. Fibromuscular dysplasia of the renal arteries usually presents with hypertension, while carotid or vertebral artery disease causes transient ischemic attacks, strokes, or dissection. Aortic dissection is rare. We present the clinical case of a patient with fibromuscular dysplasia with type B aortic dissection.
Fibromuscular dysplasia (FMD) is systemic angiopathy neither inflammatory nor atherosclerotic of unknown etiology [1]. More prevalent among young women, it can affect multiple branches of the aorta including the visceral arteries, although the most commonly affected are renal and carotid arteries. The prevalence of symptomatic renal involvement in FMD is 14/1000, and possibly half for cervicocranial [2]. The most typical presentation is arterial hypertension, but the manifestations may be different according to other arterial branches being affected, such as celiac, mesenteric, liver, spleen, iliac or coronary [3].
We report the case of a patient diagnosed with FMD with involvement of several visceral arteries admitted with chest pain secondary to a type B Stanford Dissection of the thoracic aorta.
A 54-year-old patient comes to the emergency room with chest pain. While resting in his sleep, wakes up feeling piercing pain in the center of the chest that radiates to the back and lower limbs. It is accompanied by sweating and increases with forced inspiration.
Medical history includes arterial hypertension, active smoking, chronic pulmonary obstructive disease, chronic peripheral predominantly demyelinating polyneuropathy without evidence of vasculitis, and peptic ulcer.
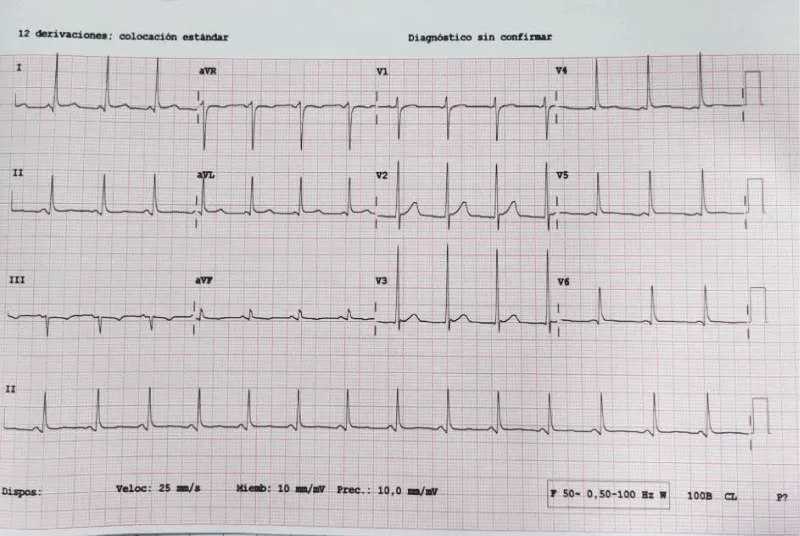
FMD is diagnosed with multiple arterial aneurysms: celiac, hepatic, renal trunk, and both common iliac arteries. In the critical care area, EKG shows a sinus rhythm at 73 bpm (Figure 1).
Figure 1: EKG on sinus rhythm, normal PR, narrow QRS, normal axis, with left ventricular hypertrophy signs.
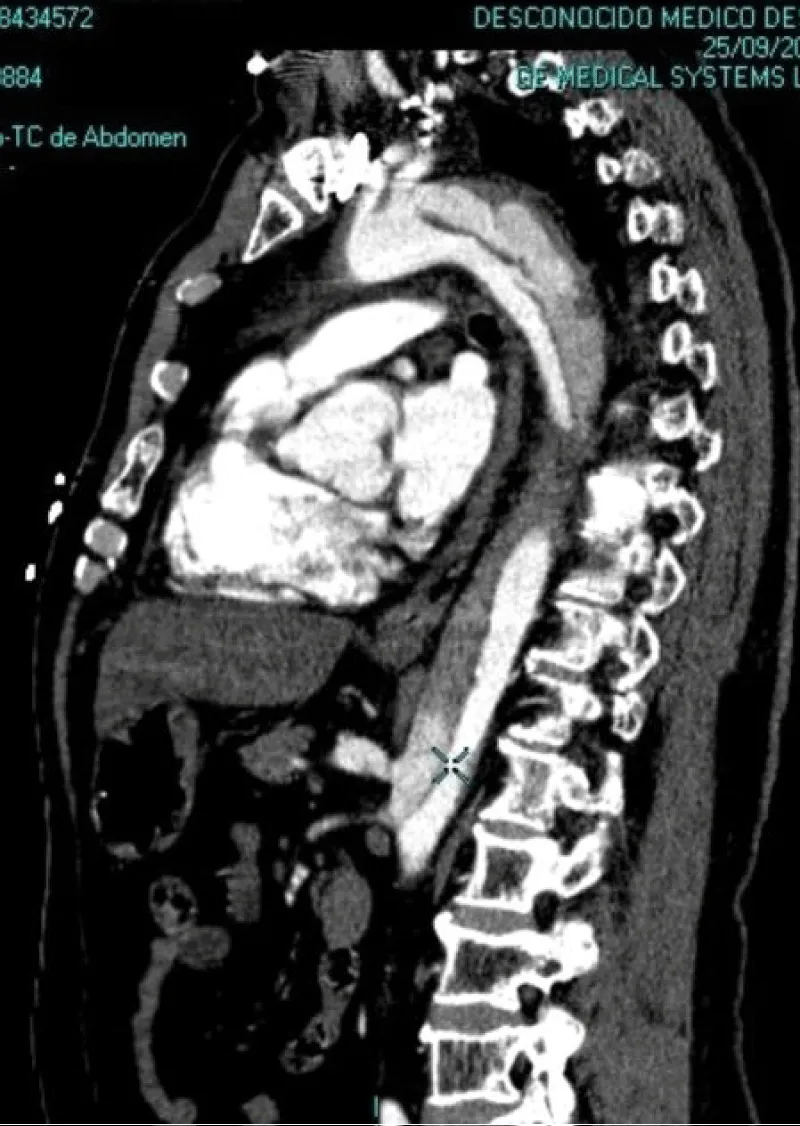
No acute ST-T abnormalities with biomarkers (troponin T and CK) repeatedly in the normal range. Normal blood count and coagulation studies. The patient is then admitted to the ICU. In the clinic of chest pain with negative electrical and enzymatic data, a CT scan is performed with intravenous contrast then unfold the existence of acute aortic syndrome Stanford type B (Figure 2). Transthoracic echocardiography was performed, showing preserved systolic function, without contractility alterations, without valvular disease but with slight left ventricular hypertrophy and slight pericardial effusion, with intimal flap distal to the left subclavian artery in the suprasternal plane.
Figure 2: Lateral CT image where it can be an observed dissection of the aortic artery, with false lumen distal to left subclavian artery to the abdominal aorta without affecting the aortic arch (Stanford type B).
Analgesic treatment is administered with morphine chloride (continuous infusion at 1 mg/h for visual analog scale lower than 3) and labetalol (continuous infusion at 2 mg/min to optimal systolic pressure values lower than 120 mmHg). During the following hours, the patient remained asymptomatic (good pain control and arterial pressure), without hypoxemia, and conserved distal pulses. No signs of peritoneal irritation and preserved diuresis.
After 48 hours of evolution abruptly appears restlessness accompanied by severe pain in the back followed by cardiac arrest. New control echocardiography was performed, showing severe pericardial tamponade with an increased false lumen in the suprasternal plane, diagnosing aortic rupture. The cardiac arrest cannot be resolved despite the immediate establishment of advanced CPR measures, existing and failing to obtain authorization for necropsy.
The cause of the FMD is unknown but a variety of factors have been implicated in the development of the disease. The possibility of genetic factors being involved is supported by the existence of familiar cases including brothers and identical twins4. Although admitted that FMD can affect anybody’s vessel, renal arteries followed by the internal carotid arteries are most commonly affected.
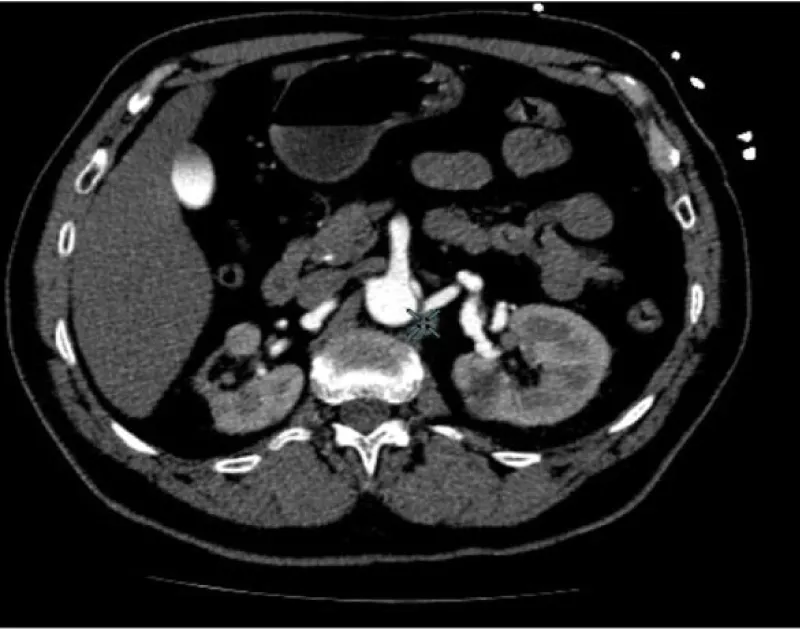
FMD is a rare disease that is often diagnosed solely based on clinical and angiographic findings. In our case, CT angiography mages show the typical appearance of a “pearl necklace” characteristic of FMD (Figure 3). Although the three arterial layers may be affected, the most frequent and responsible of the classical radiological image is the medial dysplasia, alternating areas of thickening and fibrosis in the middle and aneurysmal dilatations with loss of the internal elastic lamina.
Figure 3: CT angiography mages show the typical appearance of the “pearl necklace” characteristic of FMD.
The involvement of the aorta is rare as it makes manifest that less than 30 cases of aortic FMD are published so far, but the dissection of the thoracic aorta as a complication of FMD is even more rare [5]. This case is the third in the literature [3,4] of FMD complicated with aortic dissection and mainly pose the fact that if a patient with FMD and dissection of the thoracic aorta that remains asymptomatic with good blood pressure control should be managed exclusively with conservative medical treatment.
The type B or type III dissections should be treated medically, as agreed by the vast majority of medical and surgical equipment. Treatment should be initially aimed at reducing the contractile force of the heart and blood pressure. The surgical indication is reserved for those patients with medical treatment failure with persistent pain, signs of progression of the dissection, central nervous system or renal failure, or visceral ischemia [8]. Despite this, the patient died suddenly while remaining asymptomatic with good blood pressure control.
The contribution of this case, in addition to its rarity, is that a disease with impaired vascular wall visceral arteries developing aortic dissection should be considered a complicated disease and therefore subsidiary of emerging endovascular treatment.
1. FMD with aortic dissection is a rare and clinically important case for clinicians.
2. It is important to individualize medical or surgical management in FMD patients based on the extent of damage, blood pressure control, or pain.
3. We must investigate the genetic alterations and involvement of the family of patients with FMD for primary prevention.
- McCormack LJ, Hazard JB, Poutasse EF. Obstructive lesions of renal artery associated with remediable hypertension. Am J Pathol. 1958; 34: 582-586.
- Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, et al. Fibromuscular dysplasia. Orphanet J Rare Dis. 2007; 2: 28. PubMed: https://pubmed.ncbi.nlm.nih.gov/17555581/
- Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004; 350: 1862-1871. PubMed: https://pubmed.ncbi.nlm.nih.gov/15115832/
- Sang CN, Whelton PK, Hamper UM, Connolly M, Kadir S, et al. Etiologic factors in renovascular fibromuscular dysplasia. A case-control study. Hypertension. 1989; 14: 472-479. PubMed: https://pubmed.ncbi.nlm.nih.gov/2680961/
- Radhi JM, McKay R, Tyrrell MJ. Fibromuscular Dysplasia of the Aorta Presenting as Multiple Recurrent Thoracic Aneurysms. Int J Angiol. 1998; 7: 215-218.
- Gatalica Z, Gibas Z, Martinez-Hernandez A. Dissecting aortic aneurysm as a complication of generalized fibromuscular dysplasia. Hum Pathol. 1992; 23: 586-588. PubMed: https://pubmed.ncbi.nlm.nih.gov/1568754/
- Ciura V, Bromley A, Wong J. A case of type A aortic dissection with underlying fibromuscular dysplasia. J Radiol Case Rep. 2011; 5: 22-28. PubMed: https://pubmed.ncbi.nlm.nih.gov/22470766/
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth Analg. 2010; 111: 279-315. PubMed: https://pubmed.ncbi.nlm.nih.gov/20664093/