More Information
Submitted: July 08, 2022 | Approved: July 21, 2022 | Published: July 22, 2022
How to cite this article: Rings L, Ntinopoulos V, Dushaj S, Hoti G, Fleckenstein P, et al. Single-center experience in sutureless aortic valve implantation using two aortic valve prostheses. J Cardiol Cardiovasc Med. 2022; 7: 056-060.
DOI: 10.29328/journal.jccm.1001134
Copyright License: © 2022 Rings L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Single-center experience in sutureless aortic valve implantation using two aortic valve prostheses
Laura Rings, Vasileios Ntinopoulos, Stak Dushaj, Gojart Hoti, Philine Fleckenstein, Omer Dzemali*# and Achim Häussler#
Department of Cardiac Surgery, City Hospital Zürich, Zurich, Switzerland
#These authors contributed equally
*Address for Correspondence: Dr. Omer Dzemali, Professor, Department of Cardiac Surgery, City Hospital Zürich, Zurich, Birmensdorferstrasse 497, 8063 Zurich, Switzerland, Email: [email protected]
Background and aim of the study: Sutureless aortic valves are used to reduce operation time. However, the stent implantation might cause postoperative ECG alterations and a higher risk of paravalvular leakage. In literature, some cases of thrombocytopenia are described after implantation. We investigated the Sorin Perceval S Sutureless Valve and the Edwards Intuity Sutureless Valve.
Material and methods: Seventy-nine patients underwent aortic valve replacement using a sutureless valve in a single center between 2015 - 2018. Thirty-seven patients received Sorin Perceval S (Group A) and 42 Edwards Intuity (Group B). Simultaneous bypass surgery was performed in 23 patients in Group A and 22 patients in Group B. We compared the groups regarding postoperative TTE and paravalvular leakage, postoperative ECG alterations, need for pacemaker implantation, postoperative platelet count, and 30-day mortality
Results: Only in Group B 2 patients had paravalvular leakage, and one was reoperated within the same hospital stay. In Group A, nine patients suffered from postoperative atrial fibrillation, and in Group B, 16 patients. Left bundle branch block (LBBB) was observed in 5 patients in Group A, and 13 patients in Group B. Two patients in Group A needed a definite pacemaker, and five patients in Group B. Tachy-Brady Syndrome and LBBB were observed more frequently in Group B as well as ECG alterations. One patient died within 30 days in Group A due to multiorgan failure. This patient was older and multimorbid compared to the average. Regarding platelet count, we saw a statistically significant decrease in both groups. There were no major bleeding complications or reoperations due to hemorrhage.
Conclusion: Our data shows that sutureless aortic valve replacement is associated with new postoperative ECG alterations, which are self-limiting in most cases. Compared to the literature pacemaker implantation rate in Group B is higher.
Aortic valve stenosis is a common disease among patients over 65 years, with a prevalence of at least 2% [1]. Current guidelines suggest that all symptomatic patients with severe aortic stenosis should be evaluated for surgical aortic valve replacement (SAVR) or transcatheter aortic valve intervention (TAVI) based on different criteria, including the EuroScore II [2]. SAVR has been the gold standard for many years and comes with low mortality in experienced institutions [3]. To compete with the reported benefits of TAVI in multimorbid patients or as a less invasive solution, SAVR must be investigated closely [4]. A promising solution is sutureless aortic valve prostheses which can lead to reduced cardiopulmonary bypass time (CPB) and reduced cross-clamping of the aorta. These valves are an alternative to traditional surgical prostheses and TAVI due to their surgical approach without actual suturing [5].
The Sorin Perceval S is a bovine aortic valve prosthesis made from pericardium tissue. It is equipped with an automated anchor which stabilizes the implantation region. This anchoring system is made out of Nitinol, a compound of Nickel and Titanium designed to carry recoverable deformations. In conclusion, the prosthesis can be compressed until it reaches its final diameter when released. Early results showed favorable hemodynamic effects and a low complication rate [6].
Another sutureless valve is the Edwards Intuity, made out of bovine pericardium tissue. It comes with a polyester sealing cloth covering the expandable balloon’s stainless steel frame. Due to excellent results in the prospective TRITON study, the Edwards Intuity valve found wide acceptance [7]. Both prostheses are widely used for aortic valve replacement via sternotomy or minimally invasive approaches such as partial upper sternotomy and right or right anterior thoracotomy. The opportunity of more minimally invasive approaches along with the shortened CPB and aortic cross-clamp time may lead surgeons to consider the advances of sutureless aortic valve prostheses as an alternative to traditional surgical prostheses. Both prostheses’ implantation techniques are fast and easily reproducible after a short learning period. In this retrospective single-center experience, we want to evaluate patients who received either the Sorin Perceval S or Edwards Intuity aortic valve prosthesis regarding mortality, paravalvular leakage (PVL), and electrocardiogram (ECG) alterations.
From January 2015 to December 2018, we performed 79 sutureless valve replacements using a sutureless aortic valve in a single center in Switzerland. Thirty-seven patients received Sorin Perceval S (Group A) and 42 Edwards Intuity (Group B) sutureless valves in the aortic position. Simultaneous coronary artery bypass grafting (CABG) was performed in 21 patients in Group A and 22 patients in Group B. All cases were elective, and all patients suffered from aortic valve stenosis. General exclusion criteria were the documented refusal of data analysis.
One certified examiner performed a transthoracic echo (TTE) routinely before discharge (seventh postoperative day ± four days).
Preoperative baseline characteristics (Table 1) and intraoperative and postoperative characteristics, including (TTE), were analyzed.
The institutional ethic committee approved the study protocol, and informed patient consent was waived because of the retrospective design (BASEC Nr. 2019-01962).
All procedures were performed by 1 of 3 surgeons trained explicitly in sutureless valve procedures. All patients received intraoperative transoesophageal echocardiography (TEE).
The majority of patients underwent a full sternotomy due to simultaneous CABG. In Group A, nine patients underwent a minimally invasive approach via partial upper sternotomy and seven through anterolateral thoracotomy. In Group B, 19 patients underwent a minimally invasive approach via partial upper sternotomy and one through anterolateral thoracotomy. Partial upper sternotomy (PUS) was performed via an inverted T sternotomy with an extension into the fourth intercostal space on the right or left side. Antero-lateral thoracotomy was performed through a 4 cm incision on the third rip midclavicular line, and in one lung ventilation, the aorta was prepared through the second intercostal space.
Cannulation for CPB was performed directly via ascending aorta in all cases. Venous cannulation was performed through the right atrium in all patients operated through partial or full sternotomy. Patients with an anterolateral thoracotomy were cannulated via the right femoral vein. CPB was adjusted to mild hypothermia. Core temperature was 28 °C in combined cases and 32 °C in isolated valve replacement. After cross-clamping, antegrade Brettschneider cardioplegia was administered.
Venting of the left ventricle was achieved via the right upper pulmonary vein.
Horizontal arteriotomy was performed, and coronary Ostia was identified. After excision of the native valve, the sutureless valve was implanted in compliance with the company’s guidelines.
Temporary ventricular and atrial pacing leads were placed on the right ventricle and the right atrium. Cardiac de-airing was performed using a needle placed in the ascending aorta. A needle-vent was placed in the ascending aorta in patients with an approach via anterolateral thoracotomy.
The median cross-clamp time was 58 min and the median CPB 88 min. All valves were assessed regarding paravalvular leakage and transvalvular gradient before decannulation of the CPB. In particular, in Group A median CPB time was 85 min, and in Group B, 58 min; the cross-clamp time was a median of 57 min in Group A and 39 min in Group B.
After extracting relevant data from our institutional database, statistical analysis was performed using StatsDirect statistical software, version 3.1 (StatsDirect Ltd, Cambridge, UK).
Numerical data were expressed as median, and nominal and categorical variables were given as absolute numbers and proportions (%). The Mann-Whitney U-test was used for the analysis of numerical data. The extended version of Fisher’s exact test (Fisher-Freeman-Halton) was used for categorical variables owing to the small numbers in some categories.
We compared the groups regarding postoperative TTE and paravalvular leakage, postoperative electrocardiogram (ECG) alterations, especially the need for pacemaker implantation, postoperative platelet count, and 30-day mortality.
The study cohort’s characteristics are summarized in Table 1. Preoperative comorbidities did not differ significantly between groups.
| Table 1: Preoperative baseline characteristics. | ||
| Group A n = 37 (Sorin Perceval S) |
Group B n = 42 (Edwards Intuity) |
|
| Age (years old) | 72.55 | 77.25 |
| BSA (m2) | 1.97 | 1.75 |
| Arterial hypertension | 31 | 23 |
| COPD | 2 | 5 |
| pAVD | 11 | 8 |
| NYHA Class (I-IV) | I = 8 II = 12 III = 17 IV = 0 |
I = 5 II = 22 III = 15 IV = 0 |
| EF % | 62.5 | 62.5 |
| EuroScore II (%) | 1.26 | 2.24 |
| Coronary artery disease | 26 | 25 |
| Status post PCI/Stent | 4 | 7 |
| Atrial fibrillation | 3 | 4 |
| BSA: Body Surface Area; COPD: Chronic Obstructive Pulmonary Disease; pAVD: peripheral Arterial Vascular Disease; NYHA: New York Heart Association; EF: Ejection Fraction; PCI: Percutaneous Coronary Intervention | ||
Regarding TTE, the preoperative median mean gradient was 42 mmHg. As expected, the postoperative reduction to a median mean gradient of 10 mmHg was statistically significant (p < 0.0001). The mean reduction was 31 mmHg. Pre- and postoperative left ventricular ejection fraction (LVEF) was analyzed, and a median difference of 4.5 % (2.5% – 5%) with a 95% CI was found. The 4% mean reduction of the LVEF is statistically significant (p < 0.0001).
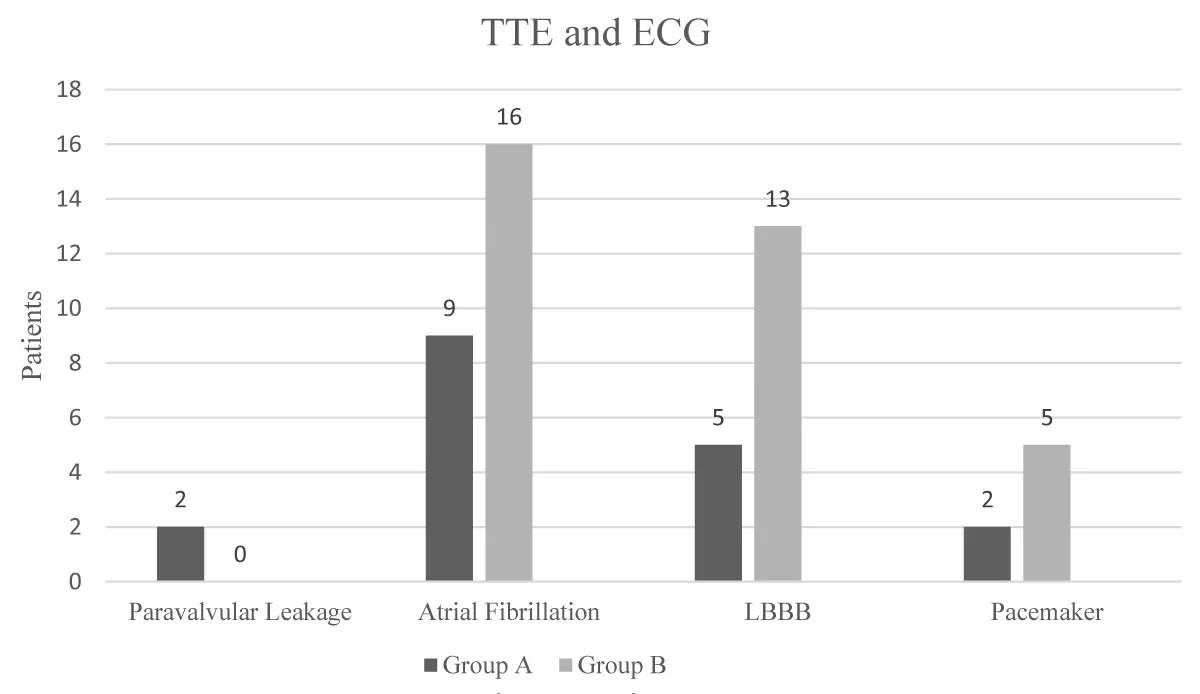
Only in Group B, did two patients have postoperative paravalvular leakage (PVL), and one was reoperated within the same hospital stay.
In Group A, nine patients suffered from postoperative atrial fibrillation, and in Group B, 16 patients. Upon discharge, only two patients in Group A needed oral anticoagulation due to persistent atrial fibrillation; in Group B, nine patients, which is statistically significant (G-square = 121.72, p < 0.0001). Left bundle branch block (LBBB) was observed in five patients in Group A and 13 patients in Group B. Two patients in Group A needed a definite transvenous pacemaker within the same hospital stay. In Group B, there were five patients for the same procedure. Tachy-Brady Syndrome and LBBB were observed more frequently in Group B (Fisher-Freeman-Halton exact p = 0.0218) as well as ECG alterations in general (Fisher-Freeman-Halton exact p = 0.0244). Results are shown in Figure 1.
Figure 1: TTE and ECG.
All patients were transferred to the intensive care unit (ICU) post-surgery. Mean stay in Group A was 32 hours and in Group B 24 hours. The total incubation time was 8 hours in Group A and 12 hours in Group B. Need for catecholamines did not differ within the groups, with a median of 20 mcg/min noradrenalin. In Group A, 1.5 red cell concentrates were transfused; in Group B, only one. Looking at the intraoperative transfusion of continuous autologous transfusion systems blood (CATS-blood), there is no apparent connection between these parameters (320 ml in Group A vs. 440 ml in Group B). Overall median hospital stay was 12.5 days in Group A and 15.5 days in Group B.
Regarding platelet count, we saw a statistically significant decrease (95% CI, p < 0.0001) in both groups (110 x 10^9/L Group A, 170 x 10^9/L Group B).
The mean platelet count nadir was 57x10^9/L in Group A and 91x10^9/L in Group B. Both nadir and overall platelet decrease evaluated at the point of hospital discharge in both groups was statistically significant (Platelet decrease: p = 0,0034 and Nadir p < 0,0001).
Only one major bleeding complication in Group A resulted in a re-thoracotomy within the first 24 hours post-surgery. This patient received three platelet concentrates. One patient in Group B received two platelet concentrates due to low platelets and a diffuse bleeding tendency. This patient was not reoperated.
One patient died within 30 days in Group A due to multi-organ failure. This patient was older and multimorbid compared to the average. His operation was a combined procedure with simultaneous CABG. His EuroScore II was 3.78% compared to the mean EuroScore II of 1.72%.
In this study, we evaluated our clinical and hemodynamic outcomes in 79 patients after sutureless valve replacement by receiving either the Sorin Perceval S or Edwards Intuity valve.
Assessing aortic cross-clamp time, we know it is an independent predictor of cardiovascular morbidity [8]. Therefore, the sutureless prostheses are at an advantage compared to conventional SAVR. Di Eusanio, et al. suggested a 20% – 40% reduction in CPB and cross-clamping time [9,10]. Regarding additional procedures, the reduced cross-clamping time with sutureless valves can also be perceived as an advantage. In our study, 43 out of 79 patients received concomitant CABG (54,4%). Hanedan, et al. published their data on sutureless aortic valve implantations and reported a CPB time of 96.51 ± 41.27 minutes, and their cross-clamping time was 60.85 ± 27.08 minutes [11]. Liakopoulos, et al. reported a cross-clamp time of 54 ± 23 minutes in patients receiving the Sorin Perceval S and 60 ± 25 minutes in their Edwards Intuity group [12]. When compared to our data, the numbers are similar or slightly higher.
Postoperative evaluation of the LVEF and PVL was made using TTE. PVL can occur due to inappropriate decalcification of the annulus, which leads to inadequate sizing or positioning of the aortic valve prosthesis. There is scientific proof that PVL correlates with poorer outcomes and is a significant predictor of one-year mortality [9]. Placing the prosthesis under direct visualization may reduce the risk of PVL. We checked for PVL intraoperatively in all patients with TEE, and no patient showed signs of it. The TTE before discharge revealed PVL in two patients in Group B, leading to one of these two being reoperated within the same hospital stay. The other patient was not reoperated due to age and comorbidities. Our patient risk stratification suggested that this patient has an acceptable hemodynamic valve performance and quality of life and does not qualify for another surgery. Shrestha et al. published their multicenter prospective pilot trial and reported only one mild PVL after Sorin Perceval S implantation, comparable to our experience with no sign of PVL in this Group [13].
As expected, we observed a significant postoperatively reduction in the aortic valve mean gradients. Our median, mean gradient of 10 mmHg is comparable to existing literature [14-16]. Large trials suggest that gradients might even be lower using TAVI [17,18]. It is difficult to compare these studies to our data because they investigated TAVR and conventional bioprostheses.
Regarding postoperative ECG alterations, we saw a statistically relevant higher amount of alterations in general in Group B. LBBB was observed in both groups but more frequent in Group B (13 patients vs. five patients), as well as atrial fibrillation and atrioventricular blockage (AV-block). The nitinol stent of the Sorin Perceval S and the expandable skirt of the Edwards Intuity may lead to higher tension in the left ventricular outflow tract (LVOT) which results in tension in the atrioventricular conduction tissue [19]. Two patients in Group A and four in Group B needed a permanent transvenous pacemaker within the same hospital stay. Our 5% postoperative pacemaker rate in Group A is lower than 6% - 11% in literature (5,6;15). After Edwards Intuity implantation, pacemaker rates vary up to 12%, so our 9.2% rate in Group B is within this range (6;15). Higher pacemaker rates can be explained by comparing the mechanism of sutureless aortic valves to TAVI [19], although further comparisons to conventional bioprostheses are needed [17].
Similar to SAVR, we experienced low 30-day mortality in this study group. Only one patient in Group A died within the same hospital stay due to multiorgan failure. This patient was older and multimorbid compared to the other patients (EuroScore II: 3,78%). Our low mortality rate might correlate with the lower EuroScore II in our patients (mean 1,72%) when looking at up to 3% - 6% in literature and higher EuroScore II between 5% - 7% (11-12;20).
We looked at postoperative platelet count and discovered a statistically significant decrease in both groups within the first five days post-surgery. There was only one major bleeding complication in Group A with resulting re-thoracotomy within the first 24 hours post-surgery. Compared to current literature, our platelet count was lower in patients receiving the Sorin Perceval S [21]. The same Group reported no exploration for bleeding. In a large controlled randomized trial (PERSIST-AVR), the standard SAVR was compared to Sorin Perceval S, and their finding regarding lower platelet count using a sutureless prosthesis aligned with our data [22].
We cannot find a clinical explanation if we look at the median hospital stay of 12 in Group A and 15.5 days in Group B. ICU stay and freedom of catecholamines were not limiting factors for hospital discharge, and there was no delay in the final medical examinations such as blood sampling or TTE. The room availability of the rehabilitation clinics might have been an issue that is difficult to trace back.
The major limitation of our study is the data from only one institution and the limited number of cases within the Group. Additionally, the retrospective design of this study without a control group is essential to note. We did not use a long-term follow-up in this descriptive study and only had short-term data. Long-term hemodynamic performance of both sutureless aortic valve prostheses needs to be obtained. Regarding low platelet count after sutureless aortic valve prosthesis, we are currently working on more detailed data from our patient collective and looking at a long-term follow-up.
Our data suggest that sutureless aortic valves are the prostheses of choice for elderly and multimorbid patients. The reduced CPB and cross-clamp time are undeniable advantages. Our institutional data shows that the postoperative hemodynamic performance is satisfactory, and the need for a permanent transvenous pacemaker is not elevated despite the expandable character of the Sorin Perceval S and Edwards Intuity. 30-day mortality was low and is comparable to SAVR. The data needs to be considered preliminary data with a relevant learning curve. We are currently evaluating the prostheses in comparison to other aortic valve prostheses.
In our institution, we see a slight advantage of the Sorin Perceval S. Both sutureless valves are fast and feasible in their preparation and implantation mechanism. However, the Sorin Perceval S showed a lower incidence of necessity for a permanent transvenous pacemaker system.
- Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999 Jul 15;341(3):142-7. doi: 10.1056/NEJM199907153410302. PMID: 10403851.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021 Oct 22;60(4):727-800. doi: 10.1093/ejcts/ezab389. Erratum in: Eur J Cardiothorac Surg. 2022 Mar 24;61(4):964. Erratum in: Eur J Cardiothorac Surg. 2022 Jun 15;62(1): PMID: 34453161.
- Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000 Mar 1;35(3):747-56. doi: 10.1016/s0735-1097(99)00584-7. PMID: 10716479.
- Jakobsen L, Terkelsen CJ, Søndergaard L, De Backer O, Aarøe J, Nissen H, Johnsen SP, Christiansen EH. Short- and Long-Term Mortality and Stroke Risk After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2018 Jan 1;121(1):78-85. doi: 10.1016/j.amjcard.2017.09.014. Epub 2017 Oct 10. Erratum in: Am J Cardiol. 2018 Feb 1;121(3):395. PMID: 29103605.
- D'Onofrio A, Salizzoni S, Filippini C, Tessari C, Bagozzi L, Messina A, Troise G, Tomba MD, Rambaldini M, Dalén M, Alamanni F, Massetti M, Mignosa C, Russo C, Salvador L, Di Bartolomeo R, Maselli D, De Paulis R, Alfieri O, De Filippo CM, Portoghese M, Bortolotti U, Rinaldi M, Gerosa G. Surgical aortic valve replacement with new-generation bioprostheses: Sutureless versus rapid-deployment. J Thorac Cardiovasc Surg. 2020 Feb;159(2):432-442.e1. doi: 10.1016/j.jtcvs.2019.02.135. Epub 2019 May 11. PMID: 31213376.
- Laborde F, Fischlein T, Hakim-Meibodi K, Misfeld M, Carrel T, Zembala M, Madonna F, Meuris B, Haverich A, Shrestha M; Cavalier Trial Investigators. Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European multicentre study (the Cavalier Trial)†. Eur J Cardiothorac Surg. 2016 Mar;49(3):978-86. doi: 10.1093/ejcts/ezv257. Epub 2015 Aug 4. PMID: 26245628.
- Kocher AA, Laufer G, Haverich A, Shrestha M, Walther T, Misfeld M, Kempfert J, Gillam L, Schmitz C, Wahlers TC, Wippermann J, Mohr FW, Roth M, Skwara A, Rahmanian P, Wiedemann D, Borger MA. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg. 2013 Jan;145(1):110-5; discussion 115-6. doi: 10.1016/j.jtcvs.2012.07.108. Epub 2012 Oct 8. PMID: 23058665.
- Ranucci M, Frigiola A, Menicanti L, Castelvecchio S, de Vincentiis C, Pistuddi V. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis. 2012 Nov;21(6):732-9. PMID: 23409353.
- Di Eusanio M, Phan K. Sutureless aortic valve replacement. Ann Cardiothorac Surg. 2015 Mar;4(2):123-30. DOI: 10.3978/j.issn.2225-319X.2015.02.06. PMID: 25870807; PMCID: PMC4384242.
- Di Eusanio M, Phan K, Berretta P, Carrel TP, Andreas M, Santarpino G, Di Bartolomeo R, Folliguet T, Meuris B, Mignosa C, Martinelli G, Misfeld M, Glauber M, Kappert U, Shrestha M, Albertini A, Teoh K, Villa E, Yan T, Solinas M. Sutureless and Rapid-Deployment Aortic Valve Replacement International Registry (SURD-IR): early results from 3343 patients. Eur J Cardiothorac Surg. 2018 Oct 1;54(4):768-773. doi: 10.1093/ejcts/ezy132. PMID: 29617925.
- Hanedan MO, Mataracı İ, Yürük MA, Özer T, Sayar U, Arslan AK, Ziyrek U, Yücel M. Early Outcomes of Sutureless Aortic Valves. Korean J Thorac Cardiovasc Surg. 2016 Jun;49(3):165-70. doi: 10.5090/kjtcs.2016.49.3.165. Epub 2016 Jun 5. PMID: 27298793; PMCID: PMC4900858.
- Liakopoulos OJ, Gerfer S, Rahmanian P, Eghbalzadeh K, Djordjevic I, Schlachtenberger G, Zeriouh M, Mader N, Choi YH, Wahlers T. Rapid Deployment Aortic Valve Replacement with the Perceval S and Intuity Elite. Thorac Cardiovasc Surg. 2021 Aug;69(5):412-419. doi: 10.1055/s-0040-1716892. Epub 2020 Oct 25. PMID: 33099764.
- Shrestha M, Folliguet T, Meuris B, Dibie A, Bara C, Herregods MC, Khaladj N, Hagl C, Flameng W, Laborde F, Haverich A. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. J Heart Valve Dis. 2009 Nov;18(6):698-702. PMID: 20099720.
- Minh TH, Mazine A, Bouhout I, El-Hamamsy I, Carrier M, Bouchard D, Demers P. Expanding the indication for sutureless aortic valve replacement to patients with mitral disease. J Thorac Cardiovasc Surg. 2014 Oct;148(4):1354-9. doi: 10.1016/j.jtcvs.2013.12.061. Epub 2014 Jan 15. PMID: 25260274.
- Laufer G, Haverich A, Andreas M, Mohr FW, Walther T, Shrestha M, Rahmanian P, Holzhey D, Roth M, Schmitz C, Schramm R, Giot C, Wahlers TCW. Long-term outcomes of a rapid deployment aortic valve: data up to 5 years. Eur J Cardiothorac Surg. 2017 Aug 1;52(2):281-287. doi: 10.1093/ejcts/ezx103. PMID: 28453629.
- Laborde F, Fischlein T, Hakim-Meibodi K, Misfeld M, Carrel T, Zembala M, Madonna F, Meuris B, Haverich A, Shrestha M; Cavalier Trial Investigators. Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European multicentre study (the Cavalier Trial)†. Eur J Cardiothorac Surg. 2016 Mar;49(3):978-86. doi: 10.1093/ejcts/ezv257. Epub 2015 Aug 4. PMID: 26245628.
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low-Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019 May 2;380(18):1706-1715. DOI: 10.1056/NEJMoa1816885. Epub 2019 Mar 16. PMID: 30883053.
- Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019 May 2;380(18):1695-1705. doi: 10.1056/NEJMoa1814052. Epub 2019 Mar 16. PMID: 30883058.
- Carrel T, Heinisch PP. History, development and clinical perspectives of sutureless and rapid deployment surgical aortic valve replacement. Ann Cardiothorac Surg. 2020 Sep;9(5):375-385. doi: 10.21037/acs-2020-surd-18. PMID: 33102176; PMCID: PMC7548211.
- Shrestha M, Fischlein T, Meuris B, Flameng W, Carrel T, Madonna F, Misfeld M, Folliguet T, Haverich A, Laborde F. European multicentre experience with the sutureless Perceval valve: clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur J Cardiothorac Surg. 2016 Jan;49(1):234-41. doi: 10.1093/ejcts/ezv040. Epub 2015 Mar 6. PMID: 25750010.
- Jiritano F, Cristodoro L, Malta E, Mastroroberto P. Thrombocytopenia after sutureless aortic valve implantation: Comparison between Intuity and Perceval bioprostheses. J Thorac Cardiovasc Surg. 2016 Dec;152(6):1631-1633. doi: 10.1016/j.jtcvs.2016.07.054. Epub 2016 Aug 4. PMID: 27575240.
- Lorusso R, Jiritano F, Roselli E, Shrestha M, Folliguet T, Meuris B, Pollari F, Fischlein T; PERSIST-AVR Investigators. Perioperative platelet reduction after sutureless or stented valve implantation: results from the PERSIST-AVR controlled randomized trial. Eur J Cardiothorac Surg. 2021 Dec 1;60(6):1359-1365. doi: 10.1093/ejcts/ezab175. PMID: 34118150.