More Information
Submitted: July 18, 2022 | Approved: August 03, 2022 | Published: August 04, 2022
How to cite this article: Kautzner J, Skala T, Fedorco M, Wunschova H, Taborsky M. Clinical utility of intracardiac echocardiography in transvenous lead extraction. J Cardiol Cardiovasc Med. 2022; 7: 061-067.
DOI: 10.29328/journal.jccm.1001135
Copyright License: © 2022 Kautzner J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Transvenous lead extraction; Intracardiac echocardiography; Cardiac implantable devices
Clinical utility of intracardiac echocardiography in transvenous lead extraction
Josef Kautzner1,2*, Tomas Skala2, Marian Fedorco2, Hanka Wunschova1 and Milos Taborsky2
1Institute for Clinical and Experimental Medicine, Prague, Czech Republic
2Palacky University Medical School, Olomouc, Czech Republic
*Address for Correspondence: Josef Kautzner, MD, PhD, FESC, Professor, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9, Prague 4, 14021, Prague, Palacky University Medical School, Olomouc, Czech Republic, Email: [email protected]
The epidemics of heart failure and an aging population resulted in an exponential rise in the use of cardiac implantable devices (CIEDs) in developed countries. This is paralleled by the increased rate of complications such as system infection or malfunction. The higher number of complications, and longer patient life expectancies, are followed by an increase in the need for lead extractions. Earlier estimates of lead extractions worldwide ranged between 10,000 to 15,000 [1,2]. Epstein and Maytin provided a substantially higher estimate – nearly 24,000 [3]. Despite significant advances in lead extraction technology and armamentarium, transvenous lead extraction (TLE) can be associated with significant morbidity and mortality. Reported major complications and mortality rates with TLE vary widely across studies, ranging from 0.4% to 7.3% [3,4]. In the European Lead Extraction ConTRolled Registry (ELECTRa), which is the perspective, voluntary registry conducted by the EHRA among specialized centers, the primary endpoint of in-hospital procedure-related major complication rate reached 1.7%, including mortality of 0.5%) [5]. Approximately two-thirds (37/58) of these complications occurred during the procedure. The most common procedure-related complications were those requiring pericardiocentesis or chest tube and/or surgical repair (1.4%). A recent analysis of the “real world” data from the National (Nationwide) Inpatient Sample in the United States identified 57,328 hospitalizations for TLE over 13 years [6]. At least one major adverse event occurred in 10.42% of procedures. Nonfatal major complications of TLE occurred in 6.3% of the population, and the all-cause in-hospital mortality rate was 4.1%.
Therefore, efforts should focus on the safety aspect of the procedure. In this respect, both pre and intraprocedural imaging appears to be critical to defining potential vascular adhesions and other abnormalities that require modification of the TLE technique. Intraprocedural imaging allows monitoring of the procedure and early recognition of complications such as cardiac perforation. Some studies reported using transesophageal echocardiography (TEE) for this purpose. However, the most suitable imaging technology is intracardiac echocardiography (ICE). The following text describes the clinical utility of ICE in the prevention and management of complications of TLE.
Phased-array intracardiac echocardiography
Despite some good experience with the rotational ultrasound-tipped catheters [7,8], this technology has limited maneuverability and depth of imaging. On the contrary, phased-array systems are very versatile and provide the whole spectrum of 2D images, including color-coded or pulsed wave Doppler. Besides, they allow adjustment of ultrasound frequency to optimize imaging quality. For all these advantages, phased-array ICE has become the dominant imaging modality in the EP laboratory.
The advantages of intracardiac echocardiography
ICE is well suited for use during intracardiac interventional procedures [9]. The main advantages include no need for general anesthesia or deep sedation, simple workflow and easy imaging view adjustment, and the proximity to cardiac structures, especially in the right heart. It allows good visualization of the relevant structures, often far better than in TEE. It is true, especially for the identification of vascular binding sites [10,11]. It can also display the regions shielded by the artificial prostheses and/or occluders. The ICE catheter is typically advanced from the femoral vein into the right atrium, right ventricle, superior vena cava, and up to the brachiocephalic vein, depending on the need for visualization.
The workflow during transvenous lead extraction
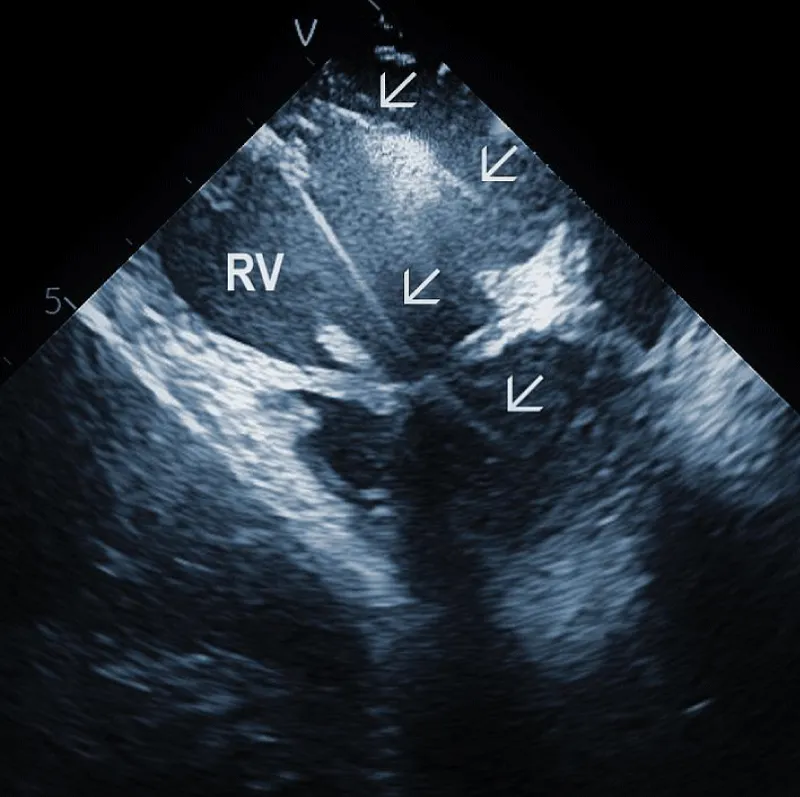
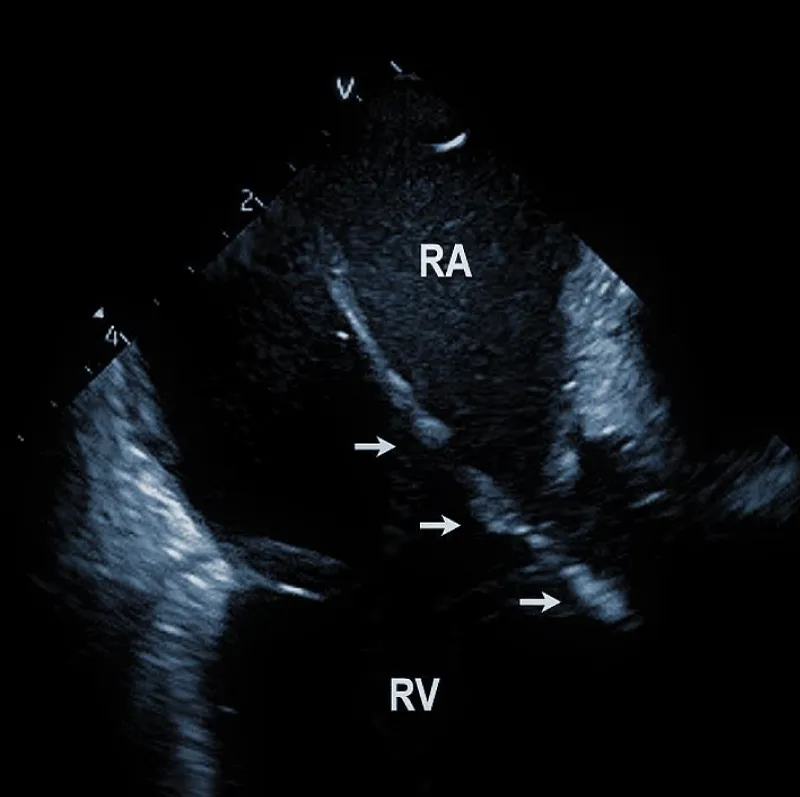
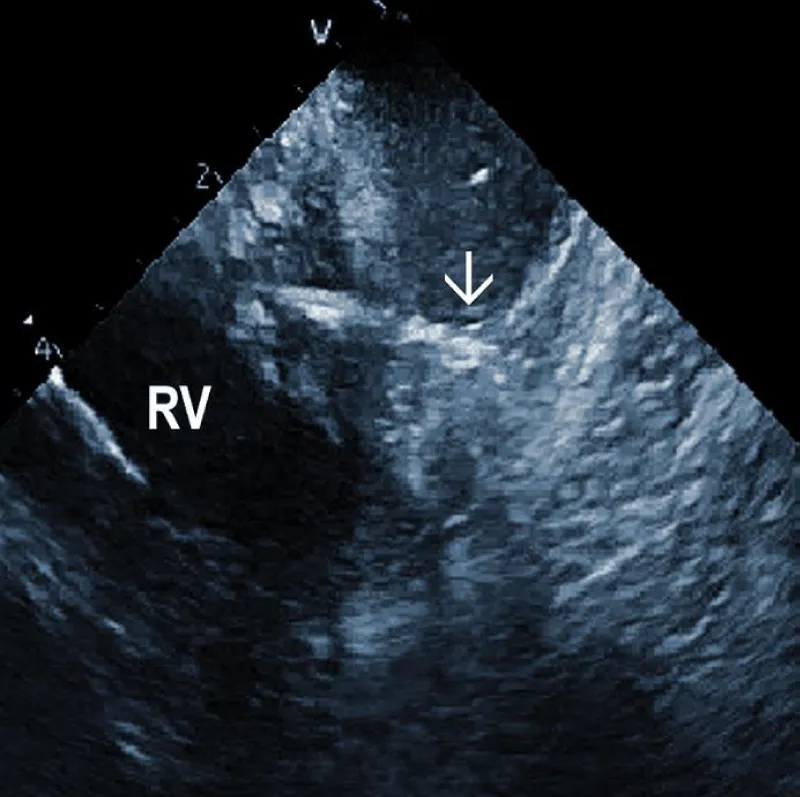
At the beginning of the procedure, ICE is introduced into the right ventricle to check and document possible pericardial effusion and left or right ventricular function. The course of the lead within the right ventricle is visible (Figure 1A). In addition, attachments of the right ventricular leads to cardiac structures could be seen, and the tip of the lead can be localized (Figure 1B).
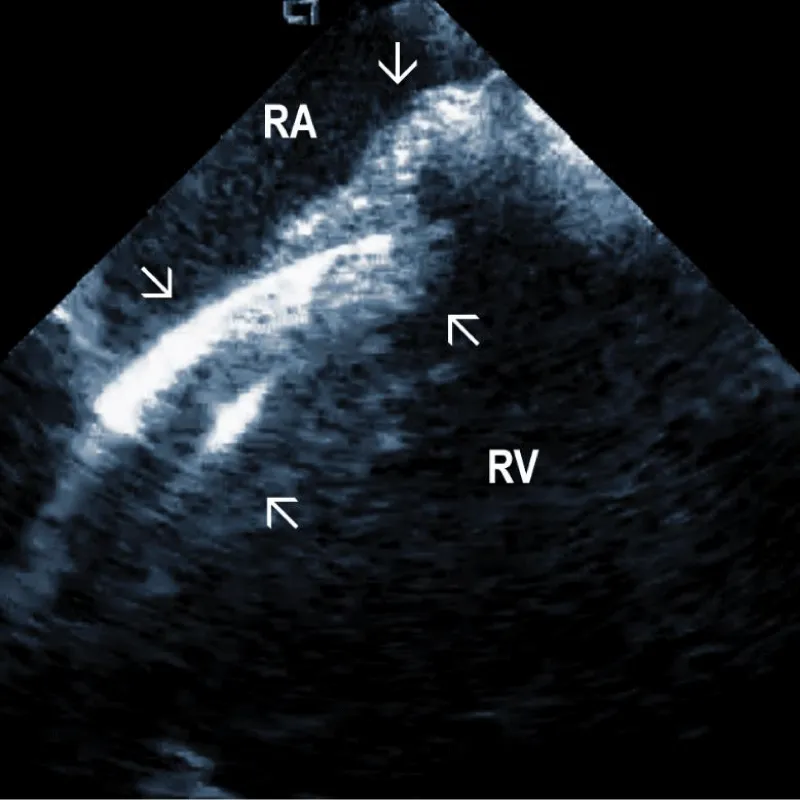
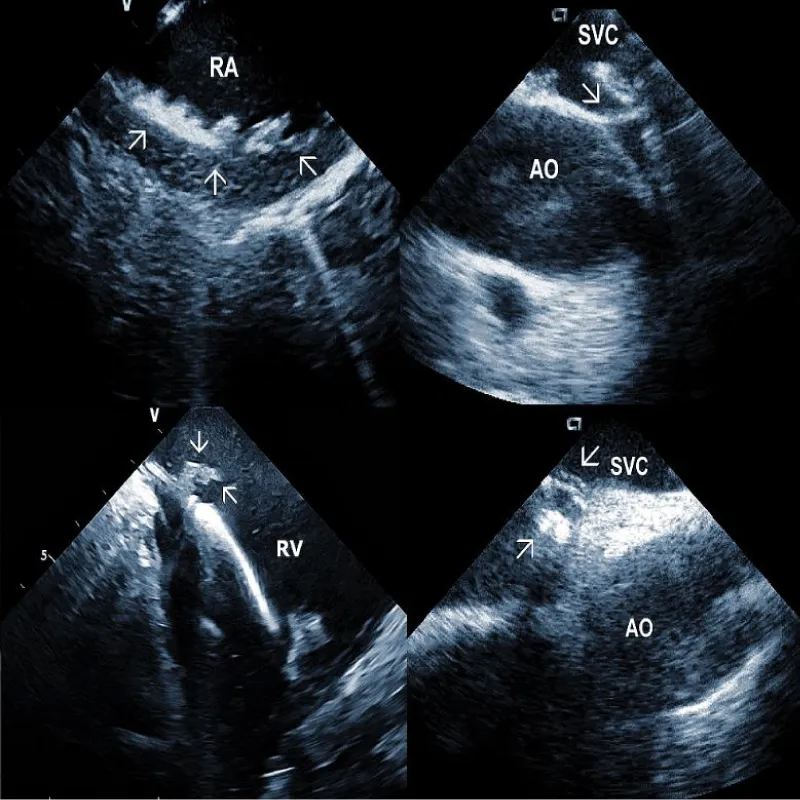
Figure 1A: The course of two pacemaker leads (arrows) within the right ventricle (RV). The ICE catheter is positioned in the basal RV.
Figure 1B: ICD lead crossing the tricuspid valve between the right atrium (RA) and right ventricle (RV). Arrows mark lead adherent echodensities. The ICE catheter is positioned in the RA.
From the right atrial position, the course of the lead (or leads) could be overlooked. This is the best view to observe the presence of tricuspid regurgitation and the existence and location of lead adherent echo densities (LAE). Positioning the catheter in the superior vena cava or higher enables assessment of the lead attachments or binding sites in this region. Subtotal or total vein occlusion could be detected at the same time.
During the procedure of TLE, the ICE catheter may be initially positioned in the SVC to monitor the advancement of mechanical or laser sheath. We prefer to place the catheter in the right atrium to monitor for right atrial or ventricular traction and for the development of pericardial bleeding. The latter could be early detected around the right atrium. Alternatively, the ICE catheter is introduced into the right ventricle to monitor right and left ventricular contours for effusion.
Finally, ICE allows a post-procedural check for the residual intracardiac mobile echo densities (ghosts) and any change in tricuspid regurgitation. The procedure is finished with the final check of pericardial effusion.
Typical findings during TLE
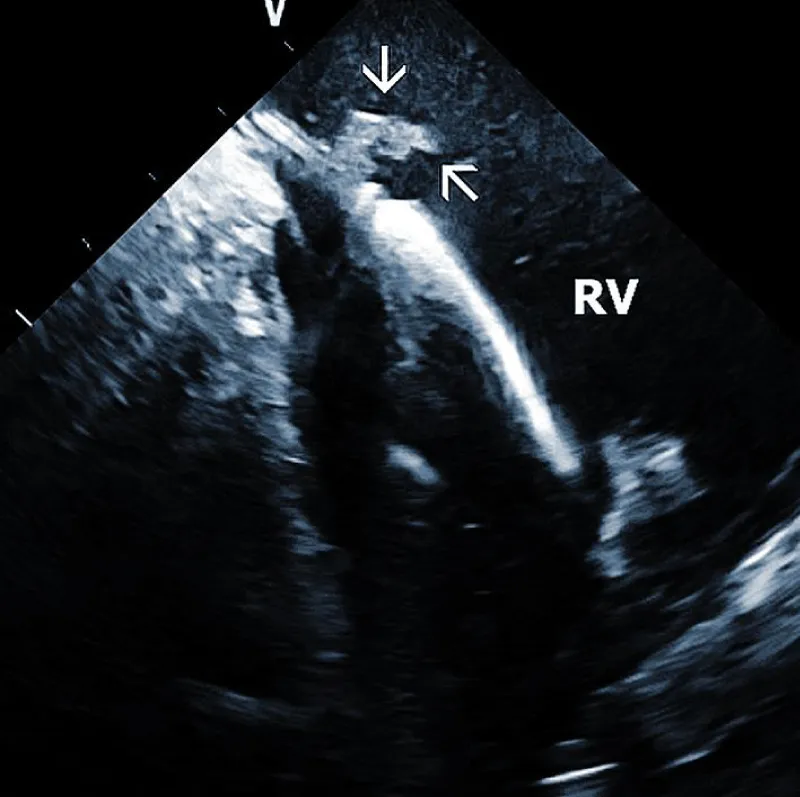
Lead adherent echo densities (LAE): ICE allows visuali-zation of the leads and echo densities adherent to them. In our experience, LAE can be detected in the majority of patients (Figure 2A). One recent study revealed LAE in 72% of cases [10]. Locations of LAE were the superior vena cava (16%), its junction with the right atrium (11%), right atrium (57%), and tricuspid annulus (16%). Finding LAE opens the question of their clinical importance. Interestingly, Ho, et al. systematically performed TEE before TLE, which was performed for non-infectious indications in 108 consecutive patients [11]. The authors reported an 18.5% incidence of lead thrombi, all of which were < 2 cm. This figure is significantly less than in the above study. This discrepancy can be explained by the higher resolution and superior maneuverability of ICE compared to TEE [12].
Figure 2A: Depiction of the lead adherent echodensity (arrows) floating on the ICD lead in the right ventricle (RV).
Therefore, ICE could be essential in patients with a suspected diagnosis of infective endocarditis since it necessitates the removal of the entire pacemaker or ICD system. Narducci et compared ICE with TEE to detect TLE in the setting-related infective endocarditis [13]. ICE identified the presence of intracardiac masses in all 58 patients (100%), whereas TEE identified the presence of ICM in only 38 patients (65%). The authors concluded that ICE is a useful technique for diagnosing intracardiac masses, thus providing improved imaging of right-sided leads and increasing the diagnostic yield compared with TEE. The pretest probability of infective endocarditis determines the incremental diagnostic value of ICE in infection of implantable devices. If the diagnosis of endocarditis is clinically rejected, even ICE does not improve the clinical decision-making process. The best diagnostic value of ICE was found in patients with a probable diagnosis of endocarditis. We recently had an ongoing pilot project focused on biopsies from TLE using a bioptome and ICE guidance. In anecdotal cases, it helped to establish the diagnosis of infective endocarditis.
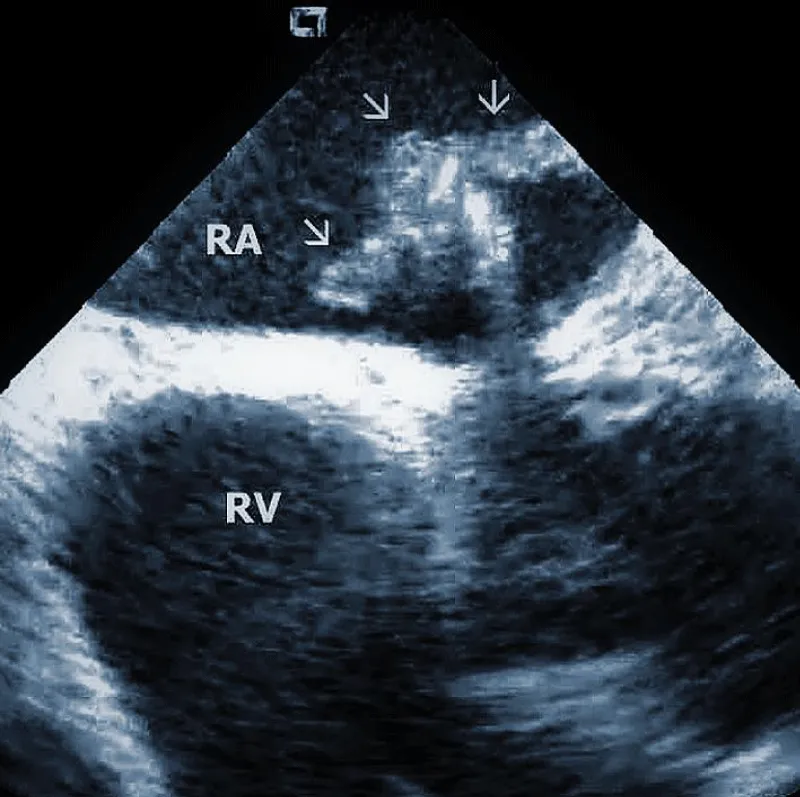
Technically speaking, the presence and size of intracardiac masses in patients suspected of infective endocarditis are critical in planning TLE procedure (Figure 2B). Large vegetations were found more common in patients with renal failure, heart failure, ICD system, and loops of the leads [14]. In another study, diabetes was related to larger vegetations, similarly younger age. Anticoagulation therapy resulted in smaller vegetations [15].
Figure 2B: Large vegetation floating on the ICD lead in the Right Atrium (RA). RV: Right Ventricle.
In patients with a definite diagnosis of lead-related infective endocarditis, the size of vegetations may determine the strategy of lead removal. Smaller vegetations < 3 cm can be extracted transvenously, while larger vegetations often require surgery. Alternatively, they may be preferably extracted by a vacuum-assisted system, usually during the same TLE procedure [16]. Some authors recommended the extraction of large vegetation placement of a Dormia basket under ICE guidance into the pulmonary artery to capture embolic material [17].
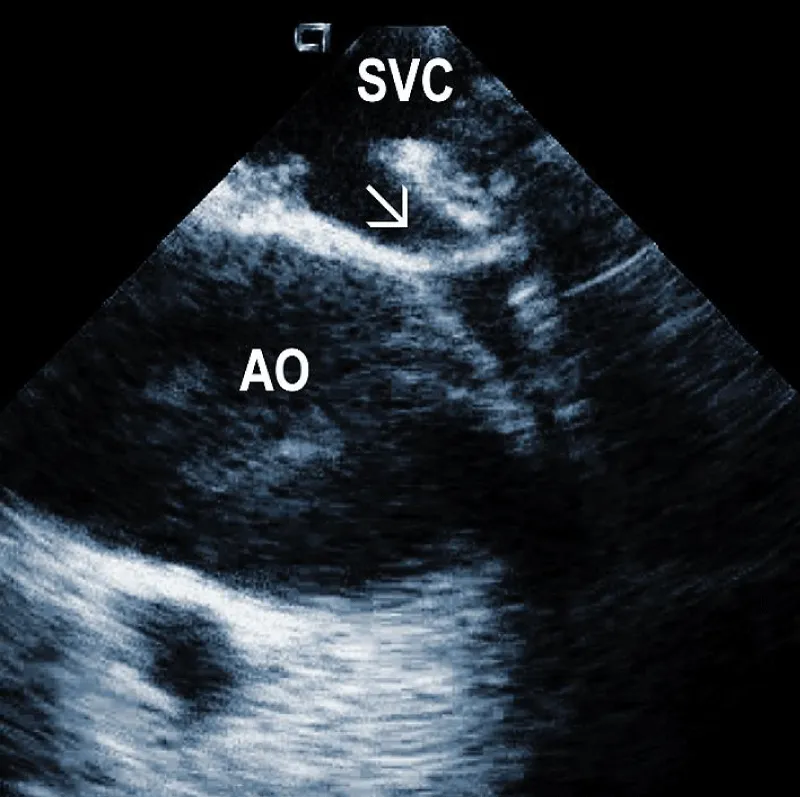
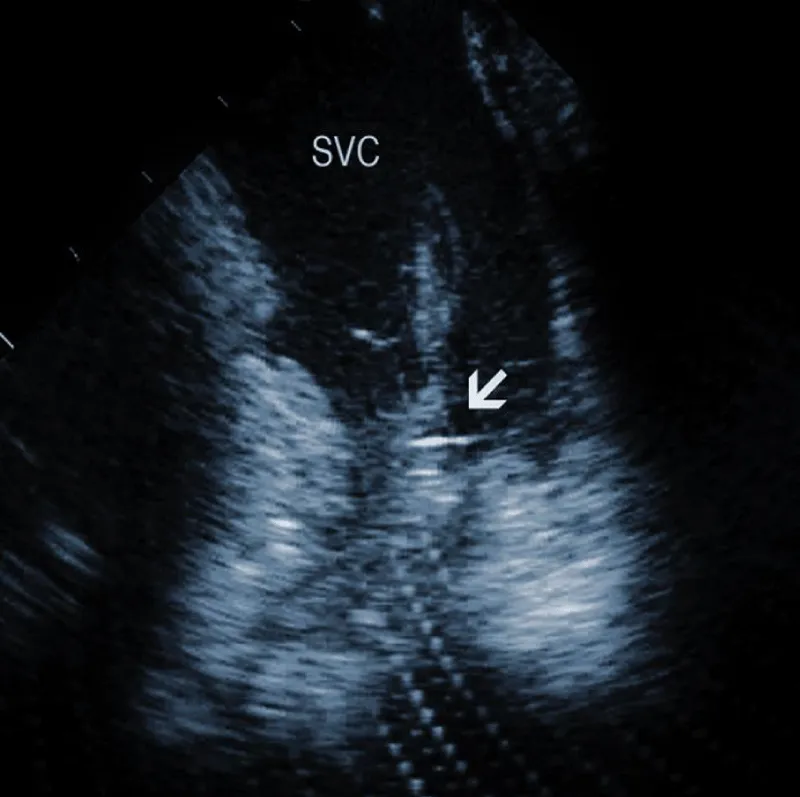
Areas of fibrous adherence: Areas of fibrous adherences or attachments (i.e., scar tissue) can be visualized as echo-dense structures along the lead course (Figure 3A). Multiple leads are usually attached by fibrous tissue together (Figure 3B). The location of fibrous adherence can be anywhere in the course of the lead [7,10]. In a study by Bongiorni, et al. [7], scar tissue was noted by ICE in the subclavian vein, innominate vein, and the right ventricle in about 80%, 68%, and 68% of cases, respectively. Sadek, et al. [10] described attachments less frequently – only in 18/50 pts (36%), predominantly intracardiac. Anecdotal cases were reported on specific locations of adhesions, such as a vulnerable “stalk” attaching the papillary muscle to the RV endocardium [18]. Notably, the fibrous attachment presence correlated with the extraction procedure’s difficulty. These patients were more likely to have a “complex” extraction procedure. Subjects who did not have evidence of lead attachment were less likely to require the advancement of the extraction sheaths past the superior vena cava and less likely to require advanced extraction tools such as snares.
Figure 3A: Fibrous adherences (arrow) attaching the lead within the superior vena cava (SVC). AO: Ascending aorta.
Figure 3B: A convolute of 2 leads attached together with fibrous tissue at the level of tricuspid valve (arrows). RA: Right Atrium; RV: Right Ventricle.
Another study, using an IVUS catheter introduced to the superior vena cava region, attempted to classify intravascular lead adherences [19]. Adherences were assorted into three categories based on three criteria: 1) lead mobility, 2) lead proximity to the vessel wall, and 3) presence of lead-onlead binding [8]. The categories were as follows: (grade 1) free-floating leads or only intermittently adjacent to the vessel wall and minimal inter-lead binding; (grade 2) leads with restricted mobility, frequently adjacent to the vessel wall with moderate lead-to-lead binding, (grade 3) immobile leads attached to the wall with significant lead-to-lead binding. Grade 1 and 2 are also classified as low grades, and grade 3 as high with elevated risk. This grading system correlated with extraction difficulty.
In addition, ICE imaging may improve the identification of patients at higher risk of superior vena cava tear. However, further studies are needed. Some operators recommend using a prophylactic vascular occlusion balloon in case of SVC adhesions [10,20]. Another option for such cases to decrease the risk of damage to the SVC is a countertraction from below [21].
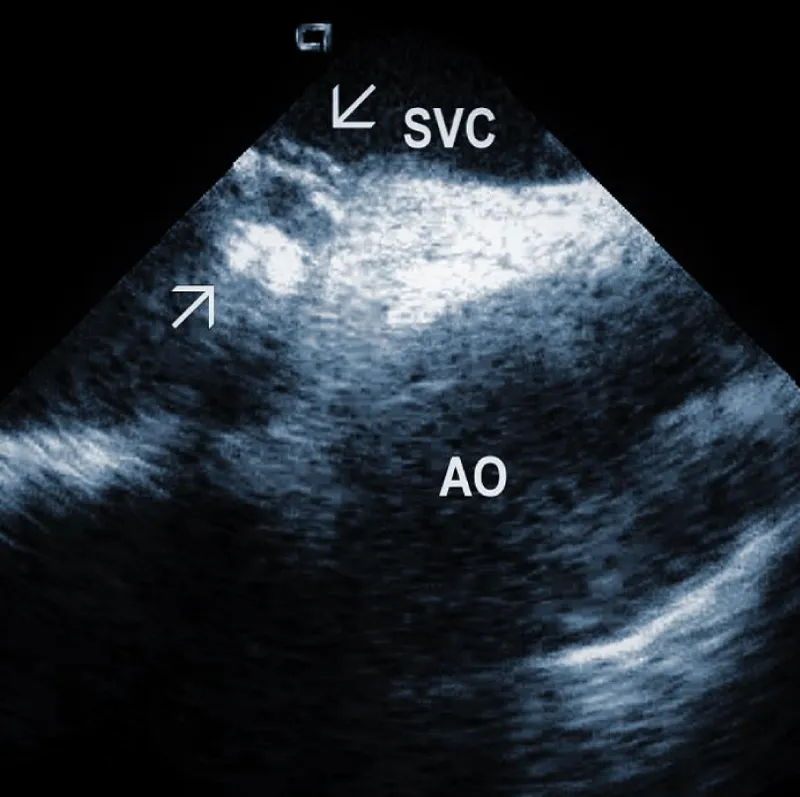
Residuals after TLE: Residual fibrotic tissue after TLE, called ghosts, has been described as a new phenomenon in cardiology (Figure 4). The presence of ghosts was first described by Rizzello, et al. using ICE [22]. Caiati, et al. studied a cohort of 40 patients using ICE before and after TLE to document the relationship between thrombotic or fibrotic reactions to the lead and with subsequent occurrence of ghosts [23]. ICE could identify thickened lead (thickness ≥ 1 mm than the vendor declared thickness in at least one lead) and fibrotic attachment to the cardiac wall in a substantial number of subjects. Thickening was noted in 25/40 patients (62%) overall, involving the atrial (2 patients (5%)) or the ventricular (9 patients (22.5%)) or both leads (14 patients (35%)). The fibrotic attachment was observed in 12/40 patients (30%). Both thickening and fibrotic attachments were significantly associated with subsequent ghosts (p < 0.001 and p = 0.002, respectively), but lead thickening had a higher prediction power. Narducci, et al. demonstrated that the presence of ghosts could be associated with a worse prognosis in device-related infective endocarditis [24]. Analyzing 217 TLE cases, the authors identified ghosts in 30 (14%) patients after TLE. In their study, endocarditis was one of the independent predictors of the presence of ghosts. Poterala, et al. detected ghosts after TLE in 19% of cases [25]. These residual fibrotic tissues were most often located along the originally implanted lead’s route. The local infection and infective endocarditis were associated with a larger number of ghosts after the removal procedure (p = 0.006). Besides frequent association with infection, detection of ghosts after TLE is also important for other reasons. Otherwise, they can be mistakenly interpreted in the echocardiographic examination as new pathological structures of unknown origin. In such a situation, the patient can be subject to unnecessary anticoagulant therapy, invasive diagnostic procedures, and/or cardiac surgery. Given the potential risks of ghosts, their presence should probably be noted on post-extraction imaging and might warrant closer post-extraction follow-up [4].
Figure 4: Ghosts within the superior vena cava (SVC) after removal of the ICD leads marked by arrows. AO: Aorta.
Damage to the tricuspid valve: Tricuspid valve damage has been reported in about 12% of cases in one series. Tricuspid regurgitation is a known complication of transvenous lead extraction [26]. This observation further underscores the importance of pre-procedure assessment of tricuspid valve function and attachments of the lead to the valve. ICE monitoring can avoid too much traction on the valve during TLE.
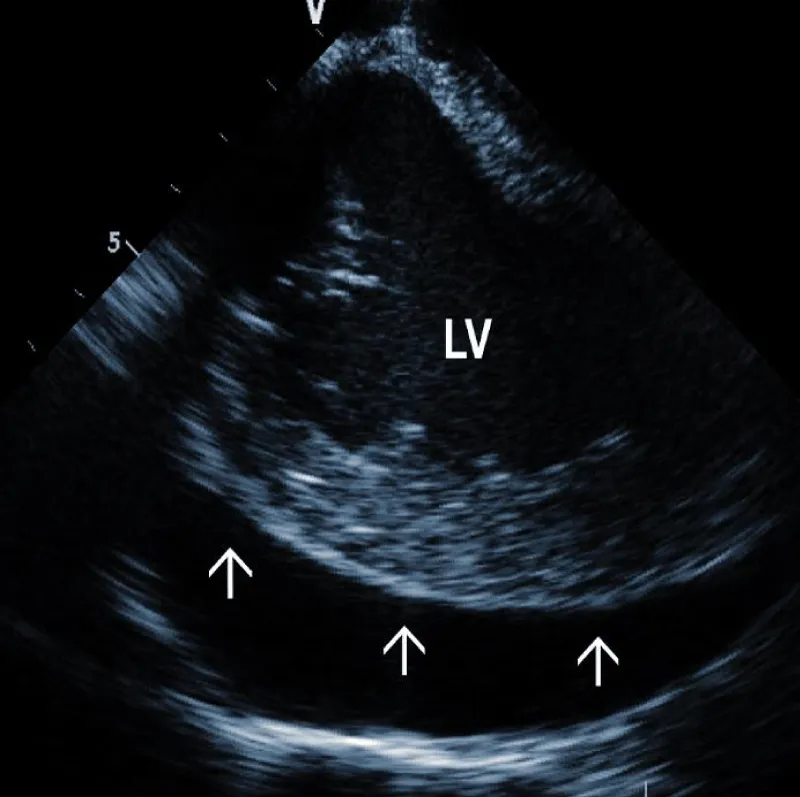
Pericardial effusion (hemopericardium): Intraproce-dural monitoring of pericardial effusion is one of the most important roles of ICE (Figure 5A). The incidence of significant pericardial effusion ranges in different studies between 0.4% - 3% [27-29]. Its immediate detection allows rapid decisions about further management. Catastrophic bleeding from the tear of the SVC may be stopped by the balloon inflation, allowing time for surgical repair. In less dramatic cases, pericardial drainage can be performed before any hemodynamic consequences occur. Our strategy is to drain every pericardial effusion that reaches more than 5 mm circumferentially. We typically use a kink-resistant 8F sheath (Arrow) to enable rapid exchange of the drainage catheter in case of blood clotting.
Figure 5A: Large separation (arrows) around the Left Ventricle (LV).
Some studies determined variables most closely associated with new-onset pericardial effusion during TLE [29]. These included three clinical factors (NYHA class III/IV, LVEF < 35%, renal impairment) and two factors related to the system itself (right-sided implant site, ≥ 2 electrodes targeted for extraction). Especially for such high-risk patients, ICE monitoring should be mandatory.
Additional echocardiographic findings: ICE can detect less frequent but important abnormalities, which may relate to TLE or subsequent lead implant. An example could be complete obstruction of the superior vena cava or the brachiocephalic vein. An additional curious finding which can be observed by ICE, is the deformation of the right ventricle during traction on the lead, often leading to hypotension [30]. The ability to visualize the lead-tissue interface during extraction (Figure 5B) and assess lead binding sites allows the operator to continuously evaluate the response to extraction maneuvers. Monitoring the progression and alignment of the laser extraction sheath with the lead is another potential utility of ICE (Figure 5C).
Figure 5B: Traction on the RV wall at the site of attachment of the ICD lead (arrow).
Figure 5C: Progression of the laser sheath (arrow) over the lead in the superior vena cava (SVC). Artifacts at the bottom are caused by application of laser beam.
Representative Figure: Illustrative examples of clinical utility of intracardiac echocardiography in transvenous lead extraction.
Assistance with snaring: In some cases, lead fragments need to be extracted by snaring them with retraction into a sheath [31]. Especially non-metallic components are not visible on X-ray, and ICE allows snaring them successfully.
Our experience
At the Department of Internal Medicine in Olomouc, 474 patients underwent TLE from 06.05.2011 until 20.11.2021. Simple traction (with locking stylet) was used in 210 cases, mechanical device Cook in 40 cases, laser sheath in 150, a combination of laser and mechanical sheath in 50, and other combined techniques in 24 cases. Extraction was unsuccessful in 5 cases, and the patients were sent to surgery (1%). Fragments of the leaders were left in 12 cases (2.5%). Serious complications occurred in 7 cases (1.5%). Asystole requiring cardiopulmonary resuscitation was observed in one subject and ventricular fibrillation in another one. ICE allowed to monitor for the development of hemopericardium in high-risk patients. Two patients presented with tamponade that was early recognized and successfully drained. However, three other patients had tamponade and despite early recognition, the bleeding was severe, and they died (0.6%). This experience prompted using wire backup for occlusion balloons to obturate SVC in high-risk cases of TLE. Based on this experience, we have started to use ICE during TLE in the other heart center in Prague.
Current experience from several centers suggests that the use of ICE during TLE procedures has many potential benefits. First, it can assess the anatomy and describe lead attachments, which can identify higher-risk procedures and the need for additional precautions. Second, it can reveal LAEs or vegetations and their size, which may influence the strategy of TLE. Third, it can help to oversee the progress of the procedure and especially, monitor the development of pericardial effusion. It can also visualize the deformation of the right ventricle during lead traction and thus, guide the procedure in a safe way. Fourth, after the procedure, ICE can detect fibrous residuals after TLE, which could be important for the follow-up of the patient. However, more experience is needed before ICE would be accepted as an obligatory imaging tool during TLE.
- Hauser RG, Katsiyiannis WT, Gornick CC, Almquist AK, Kallinen LM. Deaths and cardiovascular injuries due to device-assisted implantable cardioverter-defibrillator and pacemaker lead extraction. Europace. 2010 Mar;12(3):395-401. doi: 10.1093/europace/eup375. Epub 2009 Nov 27. PMID: 19946113; PMCID: PMC2825385.
- Maytin M, Epstein LM, Henrikson CA. Lead extraction is preferred for lead revisions and system upgrades: when less is more. Circ Arrhythm Electrophysiol. 2010 Aug;3(4):413-24; discussion 424. doi: 10.1161/CIRCEP.110.954107. PMID: 20716723.
- Epstein LM, Maytin M. Strategies for Transvenous Lead Extraction Procedures. J Innov Card Rhythm Manag. 2017 May 15;8(5):2702-2716. doi: 10.19102/icrm.2017.080502. PMID: 32494448; PMCID: PMC7252922.
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, Cha YM, Clancy J, Deharo JC, Ellenbogen KA, Exner D, Hussein AA, Kennergren C, Krahn A, Lee R, Love CJ, Madden RA, Mazzetti HA, Moore JC, Parsonnet J, Patton KK, Rozner MA, Selzman KA, Shoda M, Srivathsan K, Strathmore NF, Swerdlow CD, Tompkins C, Wazni O. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017 Dec;14(12):e503-e551. doi: 10.1016/j.hrthm.2017.09.001. Epub 2017 Sep 15. Erratum in: Heart Rhythm. 2021 Oct;18(10):1814. PMID: 28919379.
- Bongiorni MG, Kennergren C, Butter C, Deharo JC, Kutarski A, Rinaldi CA, Romano SL, Maggioni AP, Andarala M, Auricchio A, Kuck KH, Blomström-Lundqvist C; ELECTRa Investigators. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur Heart J. 2017 Oct 21;38(40):2995-3005. doi: 10.1093/eurheartj/ehx080. PMID: 28369414.
- Hosseini SM, Rozen G, Kaadan MI, Galvin J, Ruskin JN. Safety and In-Hospital Outcomes of Transvenous Lead Extraction for Cardiac Implantable Device-Related Infections: Analysis of 13 Years of Inpatient Data in the United States. JACC Clin Electrophysiol. 2019 Dec;5(12):1450-1458. doi: 10.1016/j.jacep.2019.08.020. Epub 2019 Oct 30. PMID: 31857046.
- Bongiorni MG, Di Cori A, Soldati E, Zucchelli G, Arena G, Segreti L, De Lucia R, Marzilli M. Intracardiac echocardiography in patients with pacing and defibrillating leads: a feasibility study. Echocardiography. 2008 Jul;25(6):632-8. doi: 10.1111/j.1540-8175.2008.00656.x. PMID: 18652009.
- Beaser AD, Aziz Z, Besser SA, Jones CI, Jameria Z, Kannan A, Upadhyay GA, Broman MT, Ozcan C, Tung R, Nayak HM. Characterization of Lead Adherence Using Intravascular Ultrasound to Assess Difficulty of Transvenous Lead Extraction. Circ Arrhythm Electrophysiol. 2020 Aug;13(8):e007726. doi: 10.1161/CIRCEP.119.007726. Epub 2020 Jul 6. PMID: 32628867.
- Kautzner J, Peichl P. Intracardiac echocardiography in electrophysiology. Herzschrittmacherther Elektrophysiol. 2007 Sep;18(3):140-6. doi: 10.1007/s00399-007-0574-9. PMID: 17891490.
- Sadek MM, Cooper JM, Frankel DS, Santangeli P, Epstein AE, Marchlinski FE, Schaller RD. Utility of intracardiac echocardiography during transvenous lead extraction. Heart Rhythm. 2017 Dec;14(12):1779-1785. doi: 10.1016/j.hrthm.2017.08.023. Epub 2017 Aug 24. PMID: 28843419.
- Ho G, Bhatia P, Mehta I, Maus T, Khoche S, Pollema T, Pretorius VG, Birgersdotter-Green U. Prevalence and Short-Term Clinical Outcome of Mobile Thrombi Detected on Transvenous Leads in Patients Undergoing Lead Extraction. JACC Clin Electrophysiol. 2019 Jun;5(6):657-664. doi: 10.1016/j.jacep.2019.01.007. Epub 2019 Feb 27. PMID: 31221351.
- Sadek MM, Cooper JM, Schaller RD. Lead-Adherent Echodensities: The Rule Rather Than the Exception! JACC Clin Electrophysiol. 2019 Jul;5(7):867. doi: 10.1016/j.jacep.2019.04.014. PMID: 31320018.
- Narducci ML, Pelargonio G, Russo E, Marinaccio L, Di Monaco A, Perna F, Bencardino G, Casella M, Di Biase L, Santangeli P, Palmieri R, Lauria C, Al Mohani G, Di Clemente F, Tondo C, Pennestri F, Ierardi C, Rebuzzi AG, Crea F, Bellocci F, Natale A, Dello Russo A. Usefulness of intracardiac echocardiography for the diagnosis of cardiovascular implantable electronic device-related endocarditis. J Am Coll Cardiol. 2013 Apr 2;61(13):1398-405. doi: 10.1016/j.jacc.2012.12.041. PMID: 23500279.
- Polewczyk A, Jachec W, Tomaszewski A, Brzozowski W, Czajkowski M, Polewczyk AM, Janion M, Kutarski A. Lead-related infective endocarditis: factors influencing the formation of large vegetations. Europace. 2017 Jun 1;19(6):1022-1030. doi: 10.1093/europace/euw121. PMID: 27358071.
- Caiati C, Pollice P, Lepera ME, Favale S. Pacemaker Lead Endocarditis Investigated with Intracardiac Echocardiography: Factors Modulating the Size of Vegetations and Larger Vegetation Embolic Risk during Lead Extraction. Antibiotics (Basel). 2019 Nov 19;8(4):228. doi: 10.3390/antibiotics8040228. PMID: 31752363; PMCID: PMC6963371.
- Rusia A, Shi AJ, Doshi RN. Vacuum-assisted vegetation removal with percutaneous lead extraction: a systematic review of the literature. J Interv Card Electrophysiol. 2019 Aug;55(2):129-135. doi: 10.1007/s10840-019-00555-6. Epub 2019 Apr 25. PMID: 31025152.
- Dello Russo A, Di Stasi C, Pelargonio G, Casella M, Bartoletti S, Narducci ML, Russo E, Innocenti E, Colombo D, Bellocci F, Tondo C. Intracardiac echocardiogram-guided use of a Dormia basket to prevent major vegetation embolism during transvenous lead extraction. Can J Cardiol. 2013 Nov;29(11):1532.e11-3. doi: 10.1016/j.cjca.2013.03.022. Epub 2013 Jun 25. PMID: 23809539.
- Maheshwari A, Desai ND, Giri J, Kobayashi T, Kolansky DM, Schaller RD. Use of Intracardiac Echocardiography During Transvenous Lead Extraction to Avoid a Catastrophic Injury. JACC Clin Electrophysiol. 2019 Jun;5(6):744-745. doi: 10.1016/j.jacep.2019.01.013. PMID: 31221364.
- Beaser AD, Aziz Z, Besser SA, Jones CI, Jameria Z, Kannan A, Upadhyay GA, Broman MT, Ozcan C, Tung R, Nayak HM. Characterization of Lead Adherence Using Intravascular Ultrasound to Assess Difficulty of Transvenous Lead Extraction. Circ Arrhythm Electrophysiol. 2020 Aug;13(8):e007726. doi: 10.1161/CIRCEP.119.007726. Epub 2020 Jul 6. PMID: 32628867.
- Wilkoff BL, Kennergren C, Love CJ, Kutalek SP, Epstein LM, Carrillo R. Bridge to surgery: Best practice protocol derived from early clinical experience with the Bridge Occlusion Balloon. Federated Agreement from the Eleventh Annual Lead Management Symposium. Heart Rhythm. 2017 Oct;14(10):1574-1578. doi: 10.1016/j.hrthm.2017.07.008. Epub 2017 Jul 14. PMID: 28716703.
- Schaller RD, Sadek MM, Cooper JM. Simultaneous lead traction from above and below: A novel technique to reduce the risk of superior vena cava injury during transvenous lead extraction. Heart Rhythm. 2018 Nov;15(11):1655-1663. doi: 10.1016/j.hrthm.2018.05.022. Epub 2018 May 24. PMID: 29803849.
- Rizzello V, Dello Russo A, Casella M, Biddau R. Residual fibrous tissue floating in the right atrium after percutaneous pacemaker lead extraction: an unusual complication early detected by intracardiac echocardiography. Int J Cardiol. 2008 Jul 4;127(2):e67-8. doi: 10.1016/j.ijcard.2007.04.047. Epub 2007 Jun 22. PMID: 17588687.
- Caiati C, Luzzi G, Pollice P, Favale S, Lepera ME. A Novel Clinical Perspective on New Masses after Lead Extraction (Ghosts) by Means of Intracardiac Echocardiography. J Clin Med. 2020 Aug 8;9(8):2571. doi: 10.3390/jcm9082571. PMID: 32784437; PMCID: PMC7465795.
- Narducci ML, Di Monaco A, Pelargonio G, Leoncini E, Boccia S, Mollo R, Perna F, Bencardino G, Pennestrì F, Scoppettuolo G, Rebuzzi AG, Santangeli P, Di Biase L, Natale A, Crea F. Presence of 'ghosts' and mortality after transvenous lead extraction. Europace. 2017 Mar 1;19(3):432-440. doi: 10.1093/europace/euw045. PMID: 27025772.
- Poterała M, Kutarski A, Brzozowski W, Tomaszewski M, Gromadziński L, Tomaszewski A. Echocardiographic assessment of residuals after transvenous intracardiac lead extraction. Int J Cardiovasc Imaging. 2020 Mar;36(3):423-430. doi: 10.1007/s10554-019-01731-5. Epub 2019 Nov 16. PMID: 31734932.
- Park SJ, Gentry JL 3rd, Varma N, Wazni O, Tarakji KG, Mehta A, Mick S, Grimm R, Wilkoff BL. Transvenous Extraction of Pacemaker and Defibrillator Leads and the Risk of Tricuspid Valve Regurgitation. JACC Clin Electrophysiol. 2018 Nov;4(11):1421-1428. doi: 10.1016/j.jacep.2018.07.011. Epub 2018 Sep 26. Erratum in: JACC Clin Electrophysiol. 2018 Dec;4(12):1649. PMID: 30466846.
- Swanton BJ, Keane D, Vlahakes GJ, Streckenbach SC. Intraoperative transesophageal echocardiography in the early detection of acute tamponade after laser extraction of a defibrillator lead. Anesth Analg. 2003 Sep;97(3):654-656. doi: 10.1213/01.ANE.0000074234.13373.E7. PMID: 12933378.
- Wazni O, Epstein LM, Carrillo RG, Love C, Adler SW, Riggio DW, Karim SS, Bashir J, Greenspon AJ, DiMarco JP, Cooper JM, Onufer JR, Ellenbogen KA, Kutalek SP, Dentry-Mabry S, Ervin CM, Wilkoff BL. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010 Feb 9;55(6):579-86. doi: 10.1016/j.jacc.2009.08.070. Erratum in: J Am Coll Cardiol. 2010 Mar 9;55(10):1055. PMID: 20152562.
- Regoli F, D'Ambrosio G, Caputo ML, Svab S, Conte G, Moccetti T, Klersy C, Cassina T, Demertzis S, Auricchio A. New-onset pericardial effusion during transvenous lead extraction: incidence, causative mechanisms, and associated factors. J Interv Card Electrophysiol. 2018 Apr;51(3):253-261. doi: 10.1007/s10840-018-0327-1. Epub 2018 Feb 23. PMID: 29476380.
- Sadek MM, Epstein AE, Cheung AT, Schaller RD. Pseudo-tamponade during transvenous lead extraction. Heart Rhythm. 2015 Apr;12(4):849-50. doi: 10.1016/j.hrthm.2014.12.016. Epub 2014 Dec 19. PMID: 25533583.
- Schaller RD, Sadek MM, Luebbert JJ, Ren JF, Marchlinski FE. Transjugular lead fragment extraction to improve tricuspid regurgitation. HeartRhythm Case Rep. 2015 Feb 13;1(3):95-98. doi: 10.1016/j.hrcr.2014.11.003. PMID: 28491521; PMCID: PMC5418555.