More Information
Submitted: September 13, 2022 | Approved: September 23, 2022 | Published: September 24, 2022
How to cite this article: Satoda M, Yusa H. A patient with pulseless ST elevation myocardial infarction caused by a very late stent thrombosis. J Cardiol Cardiovasc Med. 2022; 7: 081-084.
DOI: 10.29328/journal.jccm.1001138
Copyright License: © 2022 Satoda M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: ECMO; CPR; Aspiration thrombectomy; STEMI; Peri-stent contrast staining
Abbreviations: STEMI: ST Elevation Myocardial Infarction; CABG: Coronary Artery Bypass Grafting; CPR: Cardiopulmonary Resuscitation; EKG: Electrocardiogram; LCx: Left Circumflex Artery; LAD: Left Anterior Descending Artery; LVEF: Left Ventricular Ejection Fraction; PSS: Peri-Stent Contrast Staining; VA-ECMO: Venoarterial Extracorporeal Membrane Oxygenation; VLST: Very Late Stent Thrombosis; L: Left Ventricular
A patient with pulseless ST elevation myocardial infarction caused by a very late stent thrombosis
Masahiko Satoda1* and Hiroaki Yusa2
1Division of Interventional Cardiology, Ayase Heart Hospital, 16-7, 2-chome Yanaka Adachiku, Tokyo, 120-0006, Japan
2Cardiovascular Surgery, Ayase Heart Hospital, Japan
*Address for Correspondence: Masahiko Satoda, MD, Division of Interventional Cardiology, Ayase Heart Hospital, 16-7, 2-chome Yanaka Adachiku, Tokyo, 120-0006, Japan, Email: [email protected]
Background: Persistent contrast staining is highly associated with stent thrombosis.
Case summary: A 75-year-old woman presented with new-onset effort angina. A coronary angiogram revealed a 90% blockage of the distal left main trunk (LMT) and a 99% blockage of the ostial left anterior descending coronary artery (LAD). A 3.0 × 18-mm CYPHER™ the stent had previously been implanted into the dominant proximal circumflex artery (LCx) in 2009 because of unstable angina. The patient developed pulseless ST elevation myocardial infarction after the withdrawal of antiplatelet therapy before a scheduled CABG. The patient recovered with VA-ECMO and PCI using aspiration thrombectomy and urgent CABG.
Discussion: This case highlighted that a preoperative patient may develop thrombosis at a previous stent site with peri-stent contrast staining and withdrawal of an antiplatelet regimen in certain settings poses an imminent risk for preoperative deterioration. A bridging strategy using intravenous PY12 inhibitor before CABG should be considered in this setting. The revascularization strategy should be selected based on coronary anatomy, hemodynamic status and baseline risk for CABG. A hybrid revascularization approach should be considered in this patient population.
Peri-stent contrast staining (PSS) is highly associated with very late stent thrombosis (VLST) [1]. We present the case of a patient with PSS who developed refractory pulseless ST-segment elevation myocardial infarction (STEMI) after withdrawal of a PY12 inhibitor prior to scheduled coronary artery bypass grafting (CABG). To our knowledge, this is the first report of such a case in the literature and the reason we presented this case here is two-fold: the first is that PSS has not been added to the watch list of high-risk angiographic features for imminent stent thrombosis even in the latest expert consensus [2]. Second, there are guidelines regarding the revascularization strategy for non-infarct-related arteries in the STEMI patient population with refractory cardiogenic shock [3,4]. However, those guidelines have not fully elaborated on the management of patient subsets, particularly when CPR takes over 30 minutes before VA-ECMO insertion or/and critical non-culprit lesions are present, as cases with cardiogenic shock were mostly excluded from clinical trials designed to ensure safety and efficacy of immediate multivessel PCI, except for the CULPRIT-SHOCK trial [5]. Patients who were administered properly performed CPR for as long as 40 - 100 mins may survive at a rate of 14% - 35% [6]. Furthermore, in the CULPRIT-SHOCK trial, only 3.5% of trial participants underwent CABG and long CPR (> 30 min) was excluded. Thus, a selection of management options including hybrid revascularization has also been discussed.
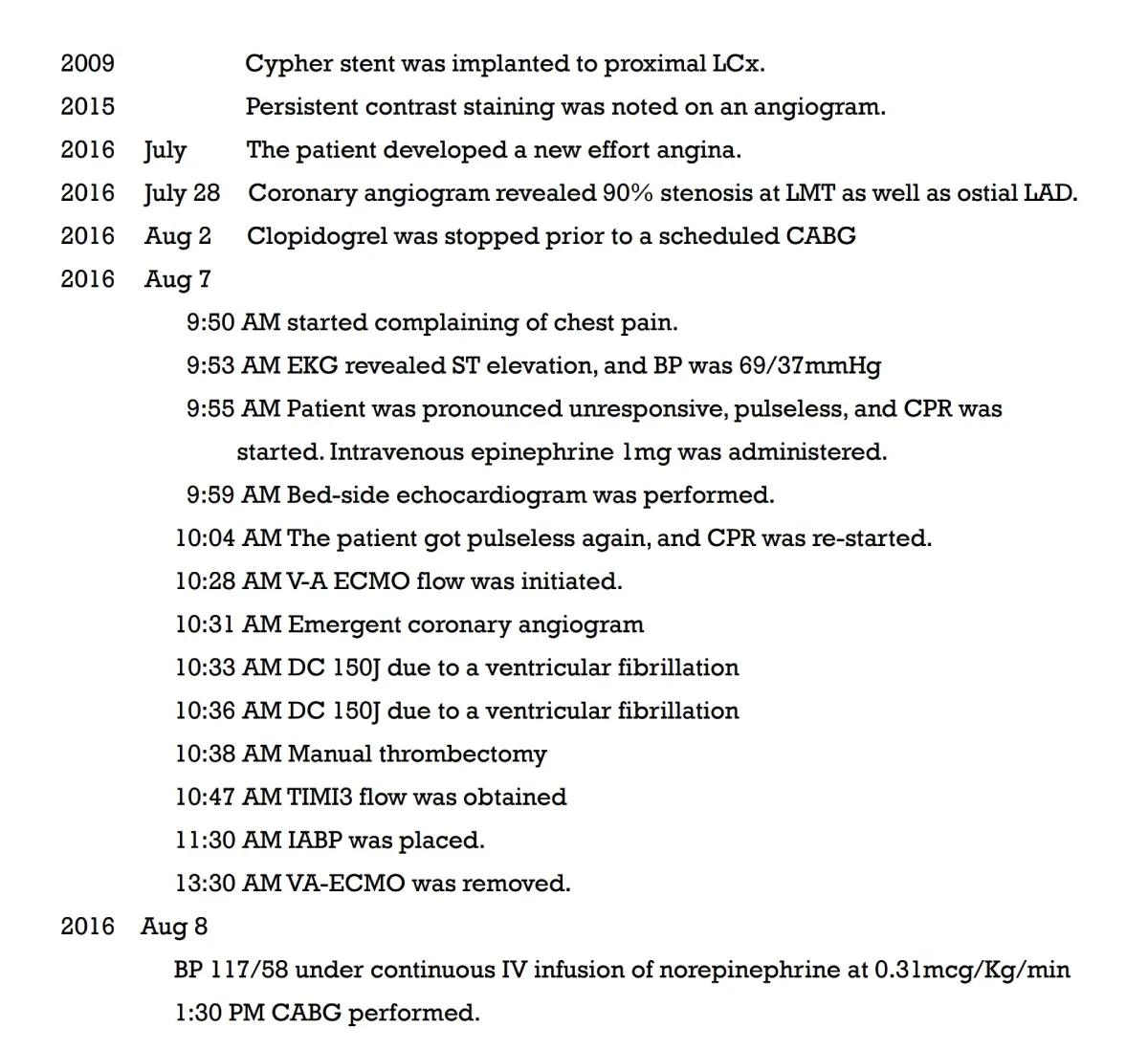
A 75-year-old woman presented with new-onset Canadian Cardiovascular Society Classification Class III angina. A coronary angiogram revealed a 90% blockage of the distal left main trunk (LMT) and a 99% blockage of the ostial left anterior descending coronary artery (LAD) (Video 1A) and (Video 1B). The Syntax Score, Society of Thoracic Surgeons (STS) score and left ventricular ejection fraction (LVEF) were 28, 1.65% and 74%, respectively. A 3.0 × 18-mm CYPHER™ stent had previously been implanted into the dominant proximal circumflex artery (LCx) in 2009 because of unstable angina (Figures 1A,1B). Although some persistent peri-stent contrast staining (PSS) was noted on an angiogram as early as June 8, 2015 (Figure 1C), a single treatment with clopidogrel for antiplatelet therapy was interrupted 7 days before an elective CABG. The patient started complaining of chest pain that corresponded with a marked ST elevation in the inferior posterior leads (Figure 2) and subsequently collapsed on August 7, 2016, in a general ward, 5 days after clopidogrel was discontinued. The patient had a history of dyslipidemia and hypertension but not diabetes, prior cerebrovascular accident, or chronic obstructive pulmonary disease. Her medication included clopidogrel (75 mg), rosuvastatin (2.5 mg), nicorandil (15 mg) and benidipine (8 mg) daily. An emergent bedside echocardiogram showed posteroinferior hypokinesia without any dissection flap in the aortic root (Video 2). Following an aggressive cardiopulmonary resuscitation (CPR) in the general ward, femorofemoral venoarterial extracorporeal membrane oxygenation (VA-ECMO) was inserted in a catheterization laboratory (Video 3). An emergent angiogram revealed a thrombotic stent occlusion of the proximal LCx (Video 4A) and (Video 4B). During angiography, the patient remained pulseless and suffered repeated bouts of ventricular fibrillation, which necessitated defibrillations. When manual aspiration thrombectomy was performed, adequate coronary flow returned instantaneously (Video 5) and (Video 6) and systolic blood pressure improved to 90 mmHg. In the end, an intra-aortic balloon pump was placed and ECMO could be tapered off the same day. The maximum creatine phosphokinase and creatine kinase-MB levels were 4398 and 269 IU/mL, respectively. The following day, the patient underwent on-pump CABG with left internal thoracic artery-LAD and saphenous vein graft-obtuse marginal anastomoses. The patient survived and recovered well without any neurological defect. As of today, LVEF is 55% and single clopidogrel therapy is ongoing. Both bypass grafts and the stent were patent (Video 7).
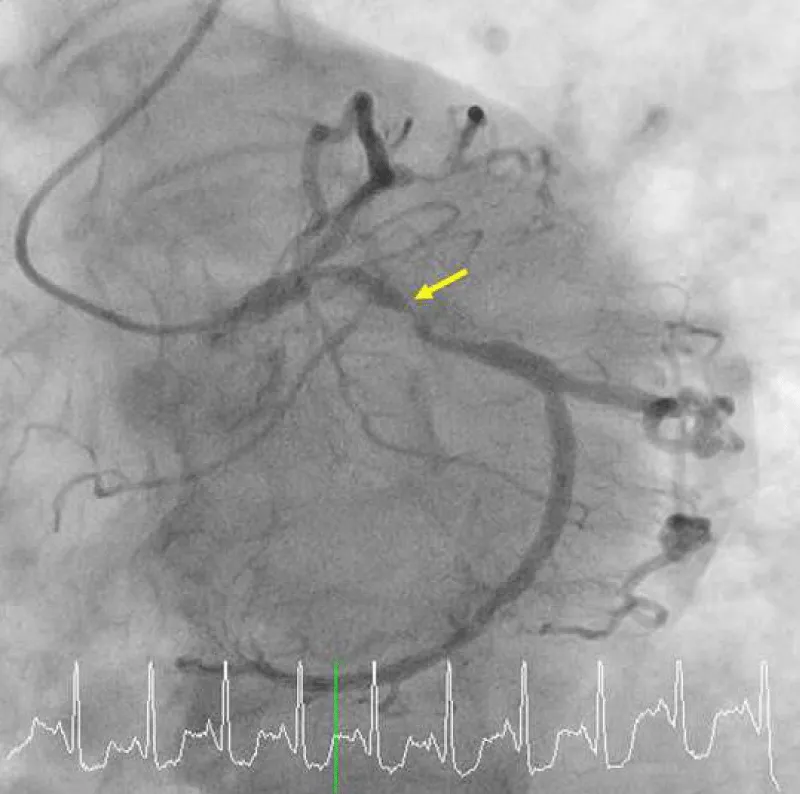
Figure 1A: A diagnostic angiogram and PCI in 2009 and a follow-up angiogram in 2015. A baseline angiogram using a 4-Fr Judkins diagnostic catheter via transradial access shows a focal 90% blockage (yellow arrow) at the dominant proximal LCx.
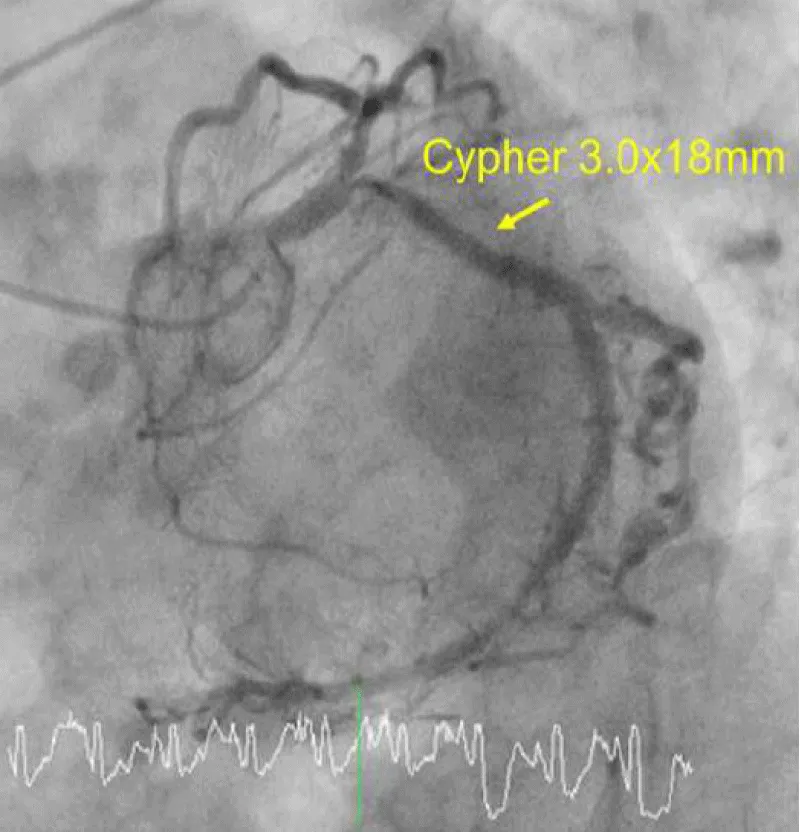
Figure 1B: A 3.0 × 18-mm CYPHER™ stent was placed without any complications such as a stent-induced dissection.
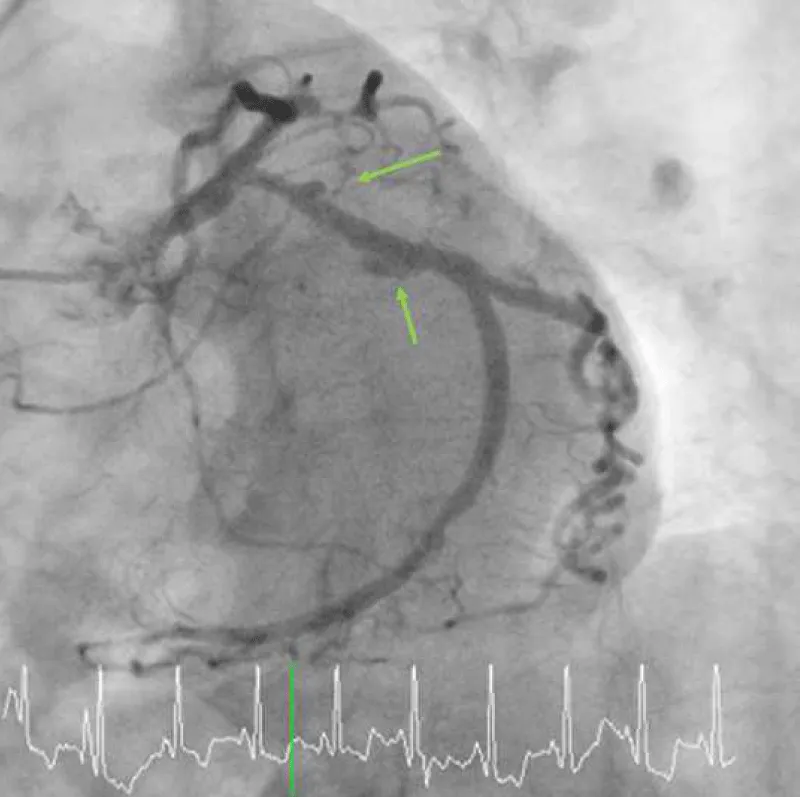
Figure 1C: Multifocal peri-stent contrast staining is depicted by green arrows, which was observed in 2015. LCx: Left Circumflex; PCI: Percutaneous Coronary Intervention.
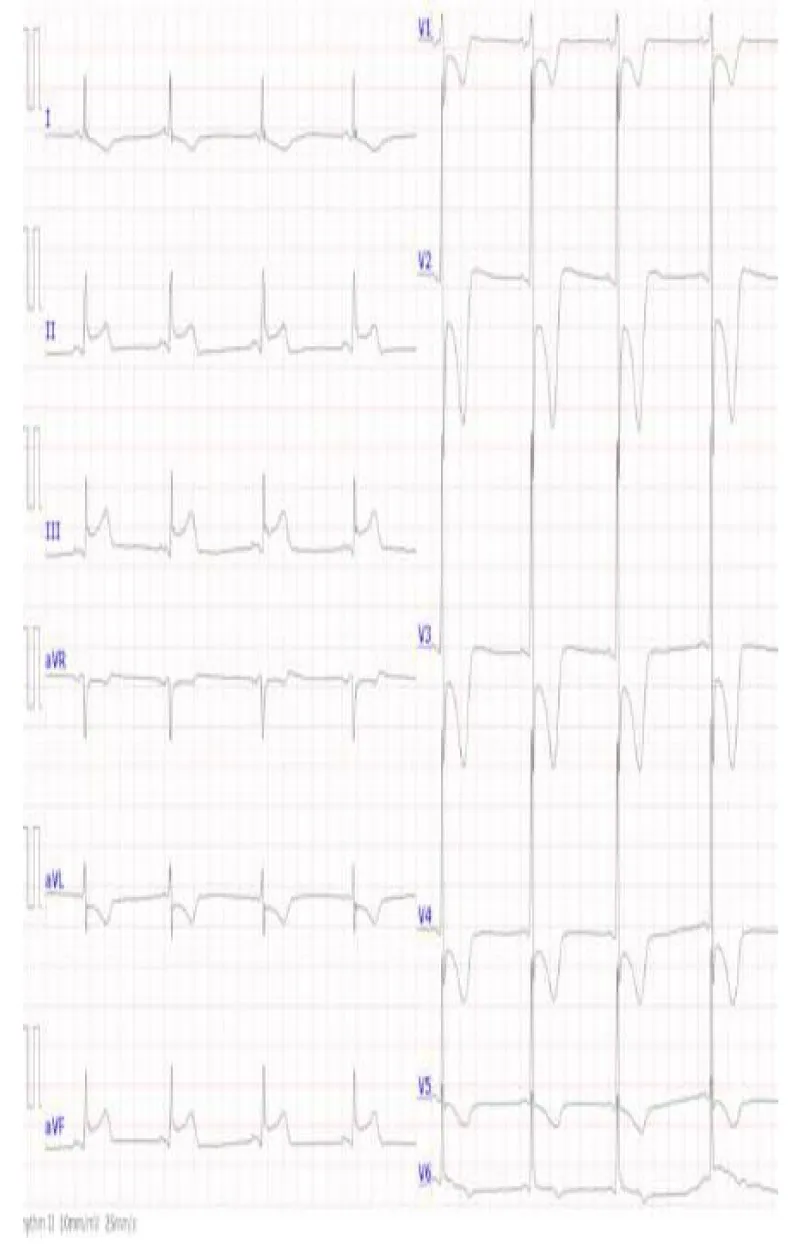
Figure 2: A 12-lead EKG at the initial presentation. ST elevations were noted at posteroinferior leads while the patient started complaining of chest pain in the general ward. At this point, the blood pressure was 69/37 mmHg. Five minutes later, the patient became unconscious and pulseless. EKG, electrocardiogram.
Timeline table was shown in Table 1.
Table 1: Timeline.
Peri-stent contrast staining of the first-generation sirolimus drug-eluting stent (CYPHER™ stent) is defined as localized angiographic dilatation (< 20%) of the vessel lumen and is associated with very late stent thrombosis (VLST) and target lesion revascularizations [1]. The frequency of VLST within 5 years of stent implantation is as high as 15% [1]. An optical coherence tomography study revealed that, in addition to stent malapposition, the presence of inner strut cavitary lesions between well-apposed stent struts, which likely represented ongoing abnormal vascular reaction and incomplete healing associated with red thrombus, partially accounted for the pathophysiology of VLST [7]. In this case, the dominant LCx was the last remaining clear vessel given the critical stenosis of the ostial LAD at baseline and thus its thrombotic occlusion lead the patient to succumb to a refractory pulseless state. Guidelines support cessation of P2Y12 inhibitors 5–7 days before elective CABG [8]. Moreover, aspirin cessation is also said to be beneficial for reducing perioperative bleeding and blood transfusion without significant adverse effects on ischemic events such as perioperative myocardial infarction or stroke [8]. For general low-thrombotic-risk CABG patients, aspirin cessation a few days before surgery doesn’t cause serious consequences [9]. In high-thrombotic-risk CABG patients, such as patients with recent stent implantation (< 3 months), a bridging strategy using intravenous PY12 inhibitor has been used in addition to continued use of aspirin with successful outcomes [2]. However, recently it has been reported that a 70-year-old female patient with recent multiple stents involving the left main trunk (< 3 months) developed ST and died on the day of operation within 3 hours after cessation of an intravenous bridging PY12 inhibitor (Cangrelor) despite continued use of aspirin [10]. Cangrelor has a rapid onset and offset of action with a short half-life (3-5 min) and platelet function returns within 30 - 60 min. Thus, Cangrelor dose titration guided by a point-of-care platelet function test during the last few hours before CABG might be necessary. Alternatively, prophylactic IABP may be considered an adjunctive option in an extremely high-thrombotic-risk cases, particularly in patients with high bleeding risk. Heparin use causes mild potentiation of platelet aggregation and the European Association for Cardio-Thoracic Surgery (EACTS) no longer supports a heparin bridge to prevent stent thrombosis [11]. Great caution should be exercised with preoperative cessation of an anti-platelet regimen in every situation to avoid perioperative bleeding. Once a stent thrombosis occurs, a prothrombotic milieu would subsequently turn non-thrombotic culprit lesions into highly thrombotic obstructive ones, leading to a catastrophic cardiogenic shock.
We advocate that it is vital to halt further procedures once the culprit lesion is recanalized with hemodynamic improvement when multiple non-culprit critical lesions are present. The reason is two-fold. First, it is best to wean off the VA-ECMO as soon as possible to reduce bleeding complications, because intrathoracic bleeding is frequently encountered after lengthy chest compression (> 30 min) [12] and such bleeding is difficult to control during heavy anticoagulation necessary to maintain a VA-ECMO circuit. Second, a lengthy PCI when pursuing perfect angiographic outcomes is often hazardous because it causes refractory slow flow and no-flow, leading to severe hemodynamic collapse and brain injury. Once this happens, prolonged ECMO support is needed until LV function recovers over several days. This is associated with blood transfusions, multiorgan failure, and complete heart block, which results in the most fatal outcomes. A manual thrombectomy is useful as it may achieve immediate recanalization and hemodynamic improvement. It will also provide an insight into thrombus characteristics thereby helping direct further procedures. A red thrombus is known to be associated with slow flow and no flow [13]. When it is present, it is advisable to consider hybrid revascularization, as further hemodynamic deterioration will not be tolerated. The timing of the CABG is crucial, as it needs to be performed at least 24 hours after the onset of cardiac arrest [14]. In a registry, the mortality risk of urgent CABG for select STEMI with cardiogenic shock patients is reported to be as low as 18.9% [15], compared to 27.0% with PCI, further supporting this approach. The true efficacy of this hybrid approach must wait for the results of an ongoing randomized clinical trial [16].
Given its association with VLST, PSS should be recognized as a high-risk marker for preoperative deterioration after the withdrawal of antiplatelet medication. In addition to low-dose aspirin, a bridging strategy using an intravenous PY12 inhibitor and/or prophylactic IABP insertion during the last few hours before CABG should be considered in the setting, particularly with high bleeding and high thrombotic risk. The use of manual thrombectomy remains a viable option as a solo procedure for managing select cases. After modulating the baseline risk of CABG by achieving hemodynamic improvement using primary PCI, such cases would likely benefit more from a timely staged CABG rather than a staged high-risk PCI.
- Imai M, Kadota K, Goto T, Fujii S, Yamamoto H, Fuku Y, Hosogi S, Hirono A, Tanaka H, Tada T, Morimoto T, Shiomi H, Kozuma K, Inoue K, Suzuki N, Kimura T, Mitsudo K. Incidence, risk factors, and clinical sequelae of angiographic peri-stent contrast staining after sirolimus-eluting stent implantation. Circulation. 2011 May 31;123(21):2382-91. doi: 10.1161/CIRCULATIONAHA.110.003459. Epub 2011 May 16. PMID: 21576652.
- Sullivan AE, Nanna MG, Wang TY, Bhatt DL, Angiolillo DJ, Mehran R, Banerjee S, Cantrell S, Jones WS, Rymer JA, Washam JB, Rao SV, Ohman EM. Bridging Antiplatelet Therapy After Percutaneous Coronary Intervention: JACC Review Topic of the Week. J Am Coll Cardiol. 2021 Oct 12;78(15):1550-1563. doi: 10.1016/j.jacc.2021.08.013. PMID: 34620413.
- Writing Committee Members, Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS Jr, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021; S0735-1097(21)06157-X
- Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019 Aug 21;40(32):2671-2683. doi: 10.1093/eurheartj/ehz363. PMID: 31274157.
- Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Savonitto S, Torremante P, Vrints C, Schneider S, Desch S, Zeymer U; CULPRIT-SHOCK Investigators. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017 Dec 21;377(25):2419-2432. doi: 10.1056/NEJMoa1710261. Epub 2017 Oct 30. PMID: 29083953.
- Yukawa T, Kashiura M, Sugiyama K, Tanabe T, Hamabe Y. Neurological outcomes and duration from cardiac arrest to the initiation of extracorporeal membrane oxygenation in patients with out-of-hospital cardiac arrest: a retrospective study. Scand J Trauma Resusc Emerg Med. 2017 Sep 16;25(1):95. doi: 10.1186/s13049-017-0440-7. PMID: 28915913; PMCID: PMC5603067.
- Tada T, Kadota K, Hosogi S, Kubo S, Ozaki M, Yoshino M, Miyake K, Eguchi H, Ohashi N, Hayakawa Y, Saito N, Otsuru S, Hasegawa D, Shigemoto Y, Habara S, Imai M, Tanaka H, Fuku Y, Oka N, Kato H, Yamamoto H, Fujii S, Goto T, Mitsudo K. Optical coherence tomography findings in lesions after sirolimus-eluting stent implantation with peri-stent contrast staining. Circ Cardiovasc Interv. 2012 Oct;5(5):649-56. doi: 10.1161/CIRCINTERVENTIONS.112.968487. Epub 2012 Sep 25. PMID: 23011265.
- Ferraris VA, Saha SP, Oestreich JH, Song HK, Rosengart T, Reece TB, Mazer CD, Bridges CR, Despotis GJ, Jointer K, Clough ER; Society of Thoracic Surgeons. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg. 2012 Nov;94(5):1761-81. doi: 10.1016/j.athoracsur.2012.07.086. PMID: 23098967.
- Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, Cooper DJ, Marasco S, McNeil J, Bussières JS, Wallace S; ATACAS Investigators of the ANZCA Clinical Trials Network. Stopping vs. Continuing Aspirin before Coronary Artery Surgery. N Engl J Med. 2016 Feb 25;374(8):728-37. doi: 10.1056/NEJMoa1507688. PMID: 26933848.
- Rossini R, Masiero G, Fruttero C, Passamonti E, Calvaruso E, Cecconi M, Carlucci C, Mojoli M, Guido P, Talanas G, Pierini S, Canova P, De Cesare N, Luceri S, Barzaghi N, Melloni G, Baralis G, Locatelli A, Musumeci G, Angiolillo DJ. Antiplatelet Therapy with Cangrelor in Patients Undergoing Surgery after Coronary Stent Implantation: A Real-World Bridging Protocol Experience. TH Open. 2020 Dec 23;4(4):e437-e445. doi: 10.1055/s-0040-1721504. PMID:
- Sousa-Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M, Dunning J, Gudbjartsson T, Linker NJ, Sandoval E, Thielmann M, Jeppsson A, Landmesser U. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018 Jan 1;53(1):5-33. doi: 10.1093/ejcts/ezx314. PMID: 29029110.
- Kaldırım U, Toygar M, Karbeyaz K. Complications of cardiopulmonary resuscitation in nontraumatic cases and factors affecting complications. Egypt J Forensic Sci 2016; 6:270–274.
- Yunoki K, Naruko T, Inoue T, Sugioka K, Inaba M, Iwasa Y, Komatsu R, Itoh A, Haze K, Yoshiyama M, Becker AE, Ueda M. Relationship of thrombus characteristics to the incidence of angiographically visible distal embolization in patients with ST-segment elevation myocardial infarction treated with thrombus aspiration. JACC Cardiovasc Interv. 2013 Apr;6(4):377-85. doi: 10.1016/j.jcin.2012.11.011. Epub 2013 Mar 20. PMID: 23523458.
- Nichols EL, McCullough JN, Ross CS, Kramer RS, Westbrook BM, Klemperer JD, Leavitt BJ, Brown JR, Olmstead E, Hernandez F, Sardella GL, Frumiento C, Malenka D, DiScipio A; Northern New England Cardiovascular Disease Study Group. Optimal Timing From Myocardial Infarction to Coronary Artery Bypass Grafting on Hospital Mortality. Ann Thorac Surg. 2017 Jan;103(1):162-171. doi: 10.1016/j.athoracsur.2016.05.116. Epub 2016 Aug 25. PMID: 27570160.
- Smilowitz NR, Alviar CL, Katz SD, Hochman JS. Coronary artery bypass grafting versus percutaneous coronary intervention for myocardial infarction complicated by cardiogenic shock. Am Heart J. 2020 Aug;226:255-263. doi: 10.1016/j.ahj.2020.01.020. Epub 2020 Mar 12. PMID: 32278440; PMCID: PMC7442583.
- https://openredcap.nyumc.org/apps/redcap/surveys/index.php?s=NLH44N9DN9