More Information
Submitted: November 24, 2022 | Approved: December 05, 2022 | Published: December 06, 2022
How to cite this article: Zaoui N, Boukabous A, Irid N, Bachir N, Terki A. Correlation between chronic inflammation of rheumatoid arthritis and coronary lesions: “About a monocentric series of 202 cases”. J Cardiol Cardiovasc Med. 2022; 7: 109-114.
DOI: 10.29328/journal.jccm.1001144
Copyright License: © 2022 Zaoui N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Patient series; Chronic inflammation; Rheumatoid arthritis; Coronary lesion; Risk factor; Rheumatoid factor
Correlation between chronic inflammation of rheumatoid arthritis and coronary lesions: “About a monocentric series of 202 cases”
Nassime Zaoui*, Amina Boukabous, Nabil Irid, Nadhir Bachir and Ali Terki
Cardiology Department, Omar Yacef Draa Ben Khedda Hospital, Tizi-Ouzou Medical University, Algeria
*Address for Correspondence: Nassime Zaoui, Cardiology Department, Omar Yacef Draa Ben Khedda Hospital, Tizi-Ouzou Medical University, Algeria, Email: [email protected]
Introduction: Cardiovascular diseases are the leading cause of death in the world, headed by coronary artery disease, which is secondary to atherosclerosis. The latter recognizes classic risk factors such as diabetes, high blood pressure, tobacco, and dyslipidemia and other less classic factors such as chronic inflammation of rheumatoid arthritis. Many studies have highlighted the correlation between this chronic inflammation and clinical coronary disease but very few have focused on the anatomical correlation.
Objective: To describe the correlation between the chronic biological inflammation of rheumatoid arthritis and anatomical coronary lesions on angiography.
Method: This observational, retrospective, single-center study, including over 10 years, of patients with rheumatoid arthritis, confirmed the EULAR 2010 criteria and presented with coronary artery disease requiring coronary angiography. Patients with missing data or in whom coronary angiography was not done were excluded (n = 14). We divided then the patients according to the existence or not of chronic inflammation to study the impact of the latter on the existence (Stenosis < 50% vs. stenosis ≥ 50%), the extent (single vs. multivessel disease), and the severity of the coronary lesions (syntax score < 32 vs. ≥ 32).
Results: 202 patients (49♂/153♀) aged between 30-75 years with a history of rheumatoid arthritis have had a coronary event requiring coronary angiography, were included; The mean ejection fraction at baseline was 57.3% +/- 5.8 (37 vs. -65%). 75% of them were ≥ 65 years old. 55% were diabetics, 61% with hypertension, 38% with dyslipidemia, and 19% were smokers. Chronic inflammation was diagnosed in 70% of them on non-specific parameters (ESR, CRP, fibrinogen, anemia, and rheumatoid factor). All patients had coronary angiography, which made it possible to identify the coronary lesions according to their existence (Stenosis < 50%: 51 patients vs. stenosis ≥ 50%: 151 patients), the extent (single: 86 patients vs. multivessel disease: 116 patients) and the severity of the coronary lesions (syntax score < 32: 142 patients vs. ≥ 32: 60 patients). Chronic inflammation of rheumatoid arthritis was correlated in bivariate and multivariate analysis (after excluding the impact of other risk factors) with the existence and extent of coronary lesions (p < 0.05) but not with their severity (p > 0.05).
Discussion: The two limitations of this work are the monocentric nature of the study and the absence of specific inflammatory parameters such as anti-CCP antibodies. Strengths are anatomical correlations and multivariate analysis. Chronic inflammation apart from any influence of the various risk factors predisposes to the existence and extent of coronary lesions (p < 0.05). The severity of coronary lesions assessed by Syntax Score was not correlated with chronic inflammation, although other studies suggest that this inflammation is the cause of complex lesions.
Interpretation: Rheumatoid arthritis is associated with an increase in cardiac morbidity and mortality. Atheromatous lesions are more frequent in those patients than the existence of classic cardiovascular risk factors would suggest. Several explanations could account for this risk: the inflammatory syndrome and its impact on the cardiovascular risk factors and the vessel and the deleterious effect of the treatments. This requires stricter screening and management of risk factors in rheumatoid arthritis.
Anti-CCP antibodies: Anti-Cyclic Citrullinated Peptide antibodies; CRP: C Reactive Protein; ECG: Electrocardiogram; EF: Ejection Fraction; ESR: Erythrocyte Sedimentation Rate; IL6: Interleukin 6; RA: Rheumatoid Arthritis; TNF alpha: Tumor Necrosis Factor-alpha; TTE: Transthoracic Echocardiography
Cardiovascular diseases are the leading cause of death in the world, headed by coronary artery disease [1], which is secondary to atherosclerosis [2]. The latter recognizes classic risk factors such as diabetes, high blood pressure, tobacco, and dyslipidemia [2] and other less classic factors such as chronic inflammation of rheumatoid arthritis [3,4].
Rheumatoid arthritis (RA) is the most common chronic inflammatory deforming rheumatism in adults with a prevalence of 0.5% to 1%, an average age of 40 years, and a female predominance (3/1) [5]. It is accompanied by a shorter life expectancy with mortality 1.5 times higher than the general population [6,7] with cardiovascular mortality of 35% to 50% [8] and risk similar to that of diabetes, which is greater with seniority and the severity of RA [9].
Many studies have highlighted the correlation between this chronic inflammation and clinical coronary disease [6,7] but very few have focused on the anatomical correlation [8,9].
This correlation was first explained by the impact of RA on the frequency and severity of classic risk factors: Tobacco (common factor), Diabetes and hypertension (Effect of inflammation: IL6 and CRP), physical inactivity, and obesity (aggravated by functional impotence) [10,11].
Then RA treatments were incriminated to explain this excess risk: NSAIDs, corticosteroids, and Janus Kinase inhibitors increase the cardiovascular risk [12], Methotrexate and hydroxychloroquine reduce the risk [13,14] while anti-TNF alpha would have a weak or neutral effect [15].
Finally, a complex pathophysiological process has been proposed to explain the impact of RA on coronary disease through the activation of T lymphocytes responsible for inflammatory synovitis by activation of synoviocytes and fibroblasts causing joint destruction and the production of pro-inflammatory cytokines (TNF Alpha, IL1 and IL6). Which activate the B lymphocytes responsible for the production of anti-cyclic citrullinated peptide antibodies (Anti CCP) leading to endothelial dysfunction with a proliferation of smooth muscle cells and the appearance of foamy macrophages. All at the origin of early atherosclerotic plaques [16-18].
It is proposed to assess the level of cardiovascular risk of these patients to multiply by 1.5 the figure obtained by the SCORE score and to include the search for asymptomatic carotid plaques by Doppler [19,20].
It is currently certain that genetic and environmental factors influence the evolution and expression of RA. Most of this work has highlighted a correlation with clinical coronary artery disease (clinical criteria) [21].
Objective
To describe the correlation between the chronic biological inflammation of rheumatoid arthritis and anatomical coronary lesions on angiography.
Study design
This is an observational, retrospective, single-center study.
Setting: The study was conducted from January 2012 to March 2022, in the cardiology department of a university hospital, using a prospective registry collecting clinical, biological, and imaging data on rheumatoid arthritis patients. Patients who were registered in our registry during this period and met the inclusion criterion for this work were enrolled in the study. Data collection lasted until March 2022.
Participants: 216 patients with a history of rheumatoid arthritis (confirmed on the EULAR 2010 criteria) presenting a coronary event (acute or chronic coronary syndrome) and requiring coronary angiography. Patients with missing data or in whom coronary angiography was not done were excluded (n = 14).
We divided then the patients according to the existence or not of chronic inflammation to study the impact of the latter on the existence (Stenosis < 50% vs. stenosis ≥ 50%), the extent (single vs. multivessel disease), and the severity of the coronary lesions (syntax score < 32 vs. ≥ 32) [22].
Variables: Symptoms and clinical examination, a precoronarographic biological assessment and an inflammatory assessment (ESR, CRP, Formula blood count, fibrinogen, and rheumatoid factor), ECG, echocardiography, and coronary angiogram were performed for all patients.
Measurement: Symptoms and clinical examination were assessed and mentioned in the patient’s medical record and the department’s registry before and after the angiography.
Biological assessments were performed in our hospital laboratory: (ESR manually on Westergren tubes, CRP and Rheumatoid factor Latex technique on Mindray automation, Fibrinogen on Biosolea 4 automat, NFS on Bachmann counter).
ECGs were performed on 12-lead devices and echocardiographic parameters were measured on a GE ultrasound machine before or immediately after the angiography. The summary of the report has been archived in the patient’s medical record and the department’s registry.
Coronary angiograms were performed on a GE Optima Cath Lab with radial 6F access and Judkins left and right sheaths, the summary of the report has been archived in the patient’s medical record and in the department’s registry.
Biases
Selection bias: To reduce these biases and make the study population as representative as possible of daily practice, we did not limit the origin of patients.
Verification bias: All patients benefited from the reference test (coronary angiography).
Interpretation bias: A double-blind coronary angiography interpretation by two interventional cardiologists was performed for all patients.
Study size: we have included consecutively all patients with the inclusion criterion (coronary event in a patient with a history of RA) from January 2012 to March 2022 bringing the total number of patients to 216 then after applying the exclusion criterion (Patients with missing data or in whom coronary angiography was not done were), 202 patients were retained in this work.
Quantitative variables: Based on the work described in the literature, we divided the patients according to the existence or not of chronic inflammation to study the impact of the latter on the existence (Stenosis < 50% vs. stenosis ≥ 50%), the extent (single vs. multivessel disease) and the severity of the coronary lesions (syntax score < 32 vs. ≥ 32).
To assess the impact of chronic inflammation on coronary lesions, patients were classified as “Inflammation +” (if at least one of the biological parameters of inflammation is positive: ESR > 50 mm/H, CRP > 10 mg/L, Fibrinogen > 5gr/L, Hb < 10 g/dL, Rheumatoid factor > 20 UI/L)) and “Inflammation –“(if all parameters are negative).
Statistical methods
All data were collected using the EPI-INFO 7 software. Results were expressed as a percentage for qualitative variables and average ± standard deviation (SD) for quantitative variables.
Bivariate analyses of coronary lesions according to the presence or absence of chronic inflammation were carried out according to the Fisher test. Then multivariate analyses of coronary lesions according to all risk factors were carried out to exclude the impact of classic risk factors.
P value < 0.05 was considered statistically significant.
Participants
216 patients were included in our study and then after the analysis of the exclusion criterion 14 patients were excluded bringing the final number of patients to 202 (Figure 1) Table 1.
Figure 1: Study flow diagram.
| Table 1: Multivariate analysis of the existence of a coronary lesion. | ||
| Risk factor | Odds ratio | p |
| Diabetes | 2.36 [1.26-3.68] | 0.0392 |
| Hypertension | 3.17 [1.19-8.42] | 0.0206 |
| Smoking | 0.76 [0.51-1.15] | 0.1930 |
| Dyslipidemia | 0.89 [0.39-2.96] | 0.1361 |
| Imflammation | 2.65 [1.45-6.72] | 0.0350 |
Descriptive data
This observational single-center study included 202 patients (49♂/153♀) aged between 30-75 years with a history of rheumatoid arthritis. having had a coronary event requiring coronary angiography; The mean ejection fraction at baseline was 57.3% +/- 5.8 (37%-65%). 75% of them were ≥ 65 years old. 55% were diabetics, 61% with hypertension, 38% with dyslipidemia, and 19% were smokers. Chronic inflammation was diagnosed in 70% of them on non-specific parameters (ESR, CRP, fibrinogen, anemia, and rheumatoid factor).
Outcome data
All patients had coronary angiography that resulted in an indication for revascularization in 70% of cases (53% by angioplasty and 17% by coronary artery bypass), while 7% of patients had lesions that could not be revascularized and 23% had no significant lesion.
The coronary angiography made it possible to identify the coronary lesions according to their existence (Stenosis < 50%: 51 patients vs. stenosis ≥ 50%: 151 patients), the extent (single: 86 patients vs. multivessel disease: 116 patients) and the severity of the coronary lesions (syntax score < 32: 142 patients vs. ≥ 32: 60 patients).
Main results
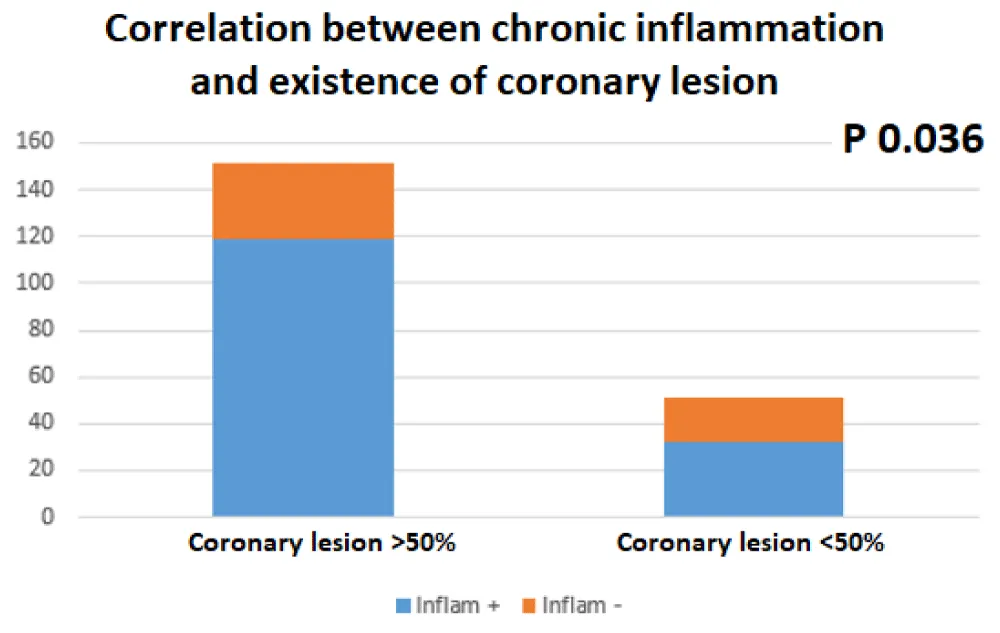
Chronic inflammation of RA was correlated in bivariate analysis with the existence of the coronary lesion (151 patients with coronary lesion≥50%: 100 of them with “inflammation +, p 0.036) sensitivity: 79%, specificity 63% (Figure 2) Table 2.
Figure 2: Correlation between RA chronic inflammation and the existence of a coronary lesion.
| Table 2: Multivariate analysis of the extent of coronary lesions. | ||
| Risk factor | Odds ratio | p |
| Diabetes | 2.88 [1.56-5.36] | 0.0298 |
| Hypertension | 1.16 [0.90-5.32] | 0.0812 |
| Smoking | 1.44 [0.79-2.63] | 0.2390 |
| Dyslipidemia | 0.92 [0.46-3.21] | 0.1401 |
| Imflammation | 3.12 [1.82-6.43] | 0.0270 |
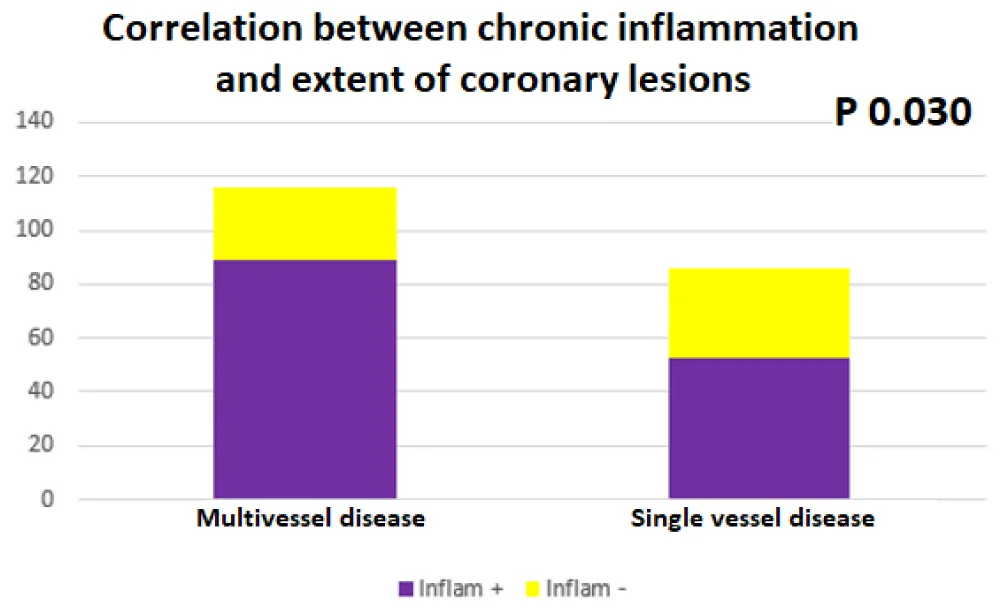
The chronic inflammation of RA was also correlated in bivariate analysis with the extent of the coronary lesions (116 patients with multivessel disease: 89 of them with “inflammation +, p 0.030), Sensitivity 77%, specificity 61% (Figure 3) Table 3.
Figure 3: Correlation between RA chronic inflammation and extent of coronary lesion.
| Table 3: Multivariate analysis of the severity of coronary lesions. | ||
| Risk factor | Odds ratio | p |
| Diabetes | 1.90 [1.01-2.26] | 0.0433 |
| Hypertension | 1.09 [0.19-13.00] | 0.9462 |
| Smoking | 2.71 [0.21-5.62] | 0.4482 |
| Dyslipidemia | 1.25 [0.36-4.37] | 0.2738 |
| Imflammation | 2.01 [0.63-3.18] | 0.0891 |
The chronic inflammation of RA was not correlated in bivariate analysis with the severity of the coronary lesions (48 patients’ syntax score ≥ 32: 39 of them with “inflammation +, p 0.085) (Figure 4).
Figure 5: Correlation between RA chronic inflammation and severity of the coronary lesion.
The multivariate analysis including all other classic risk factors (Diabetes, hypertension, smoking, dyslipidemia) to the chronic inflammation of the RA confirms the existence of a good correlation between the latter and the existence and extent of coronary lesions (p respectively at 0.035 and 0.027 and correlation coefficient of 0.64 and 0.67) but not with their severity (p > 0.089).
Highlights of the study
Strengths of this study are the anatomical correlations and the multivariate analysis.
Limitations
The two limitations of this work are the monocentric nature of the study and the absence of specific inflammatory parameters such as anti-CCP antibodies.
Key results
Chronic inflammation, apart from any influence of the various risk factors predisposes to the existence and extent of coronary lesions (p < 0.05). The severity of coronary lesions assessed by Syntax Score was not correlated with chronic inflammation, although other studies suggest that this inflammation is the cause of complex lesions.
This work will be supplemented by a multicentric prospective work according to the same scheme but taking into consideration more specific inflammatory factors such as anti-CCP antibodies. First, a correlation will have to be found then thanks to the ROC curves we will choose the best rate, which gives a good sensitivity/specificity, from this value and likelihood ratios can be calculated, thus modifying the pre-test probability and consequently the decision in a patient with RA who presents with a chronic coronary syndrome.
Interpretation
Rheumatoid arthritis is associated with an increase in cardiac morbidity and mortality. Atheromatous lesions are more frequent in those patients than the existence of classic cardiovascular risk factors would suggest. Several explanations could account for this risk: the inflammatory syndrome and its impact on the cardiovascular risk factors and the vessel wall and the deleterious effect of the treatments. This requires stricter screening and management of risk factors in rheumatoid arthritis.
Patients with predictors of poor progress should have the more intensive initial treatment and longer follow-up.
Generalisability
The results of this work are very promising but should be confirmed by a greater prospective multicentric study.
What we know
- Rheumatoid arthritis (RA) is the most common chronic inflammatory deforming rheumatism in adults with a prevalence of 0.5% to 1%, an average age of 40 years, and a female predominance (3/1).
- It is accompanied by a shorter life expectancy with mortality 1.5 times higher than the general population with cardiovascular mortality of 35% to 50% and risk similar to that of diabetes.
What this study adds
- Rheumatoid arthritis is associated with more frequent and more diffuse coronary lesions.
- This requires stricter screening and management of risk factors in rheumatoid arthritis patients.
Ethics committee
The hospital’s ethics committee has given its consent to carry out this study and share the results.
Ethics approval and consent to participate
The hospital’s ethics committee has given its consent to carry out this study and share the results under the number 102/22 delivered on June 2022.
Consent for publication
The hospital’s ethics committee consented to the sharing and publication of data and results.
Availability of data and material
The datasets used and analyzed during this work are available from the corresponding author upon reasonable request.
Authors’ contributions
NZ was responsible for the design of the study, participated in the realization of echocardiographies and coronarographies, interpreted the results, and participated in the writing of the manuscript.
AB participated in the analysis and interpretation of the results and the realization of echocardiographies.
NI participated in the realization of echocardiographies and the writing of the manuscript.
NB participated in the realization of echocardiographies and coronarographies and in the writing of the manuscript.
AT participated in the realization of echocardiographies and carried out the analysis and statistical tests.
We thank our paramedics who participated in the explorations carried out in this study and our medical secretaries who ensured the archiving of the patient’s data.
- Karlinsky A, Kobak D. The World Mortality Dataset: Tracking excess mortality across countries during the COVID-19 pandemic. medRxiv. 2021 Jun; 4:2021.01.27.21250604.
- Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med. 2014 Sep;127(9):807-12. doi: 10.1016/j.amjmed.2014.04.015. Epub 2014 May 5. PMID: 24811552.
- Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003 Mar 11;107(9):1303-7. doi: 10.1161/01.cir.0000054612.26458.b2. PMID: 12628952.
- Hajar R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views. 2017 Jul-Sep;18(3):109-114. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_106_17. PMID: 29184622; PMCID: PMC5686931.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006 Dec;36(3):182-8. doi: 10.1016/j.semarthrit.2006.08.006. Epub 2006 Oct 11. PMID: 17045630.
- Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005 Mar;52(3):722-32. doi: 10.1002/art.20878. PMID: 15751097.
- Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008 Dec 15;59(12):1690-7. doi: 10.1002/art.24092. PMID: 19035419.
- Gabriel SE. Why do people with rheumatoid arthritis still die prematurely? Ann Rheum Dis. 2008 Dec;67 Suppl 3(Suppl 3):iii30-4. doi: 10.1136/ard.2008.098038. PMID: 19022810; PMCID: PMC2830861.
- Peters MJ, van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, Visser M, Stehouwer CD, Dekker JM, Nijpels G, Heine R, Dijkmans BA, Nurmohamed MT. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009 Nov 15;61(11):1571-9. doi: 10.1002/art.24836. PMID: 19877093.
- Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A. Méta-analyse des facteurs de risque traditionnels cardiovasculaires dans la polyarthrite rhumatoïde. Revue du Rhumatisme, 2011 ;78(3):245-50.
- Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One. 2015 Feb 17;10(2):e0117952. doi: 10.1371/journal.pone.0117952. PMID: 25689371; PMCID: PMC4331556.
- Solomon DH, Husni ME, Wolski KE, Wisniewski LM, Borer JS, Graham DY, Libby P, Lincoff AM, Lüscher TF, Menon V, Yeomans ND, Wang Q, Bao W, Berger MF, Nissen SE; PRECISION Trial Investigators. Differences in Safety of Nonsteroidal Antiinflammatory Drugs in Patients With Osteoarthritis and Patients With Rheumatoid Arthritis: A Randomized Clinical Trial. Arthritis Rheumatol. 2018 Apr;70(4):537-546. doi: 10.1002/art.40400. PMID: 29266879.
- Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002 Apr 6;359(9313):1173-7. doi: 10.1016/S0140-6736(02)08213-2. PMID: 11955534.
- Sharma TS, Wasko MC, Tang X, Vedamurthy D, Yan X, Cote J, Bili A. Hydroxychloroquine Use Is Associated With Decreased Incident Cardiovascular Events in Rheumatoid Arthritis Patients. J Am Heart Assoc. 2016 Jan 4;5(1):e002867. doi: 10.1161/JAHA.115.002867. PMID: 26727968; PMCID: PMC4859400.
- Nakayamada S, Kubo S, Iwata S, Tanaka Y. Recent Progress in JAK Inhibitors for the Treatment of Rheumatoid Arthritis. BioDrugs. 2016 Oct;30(5):407-419. doi: 10.1007/s40259-016-0190-5. Erratum in: BioDrugs. 2016 Oct;30(5):483. PMID: 27577235.
- Galarraga B, Khan F, Kumar P, Pullar T, Belch JJ. C-reactive protein: the underlying cause of microvascular dysfunction in rheumatoid arthritis. Rheumatology (Oxford). 2008 Dec;47(12):1780-4. doi: 10.1093/rheumatology/ken386. Epub 2008 Oct 14. PMID: 18854346.
- Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol. 2013 Aug;64(4):409-21. PMID: 24101387.
- Sandoo A, Veldhuijzen van Zanten JJ, Metsios GS, Carroll D, Kitas GD. Vascular function and morphology in rheumatoid arthritis: a systematic review. Rheumatology (Oxford). 2011 Nov;50(11):2125-39. doi: 10.1093/rheumatology/ker275. Epub 2011 Sep 16. PMID: 21926155.
- Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, Kvien TK, Dougados M, Radner H, Atzeni F, Primdahl J, Södergren A, Wallberg Jonsson S, van Rompay J, Zabalan C, Pedersen TR, Jacobsson L, de Vlam K, Gonzalez-Gay MA, Semb AG, Kitas GD, Smulders YM, Szekanecz Z, Sattar N, Symmons DP, Nurmohamed MT. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017 Jan;76(1):17-28. doi: 10.1136/annrheumdis-2016-209775. Epub 2016 Oct 3. PMID: 27697765.
- Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, Ibsen H, Torp-Pedersen C, Olsen MH. Risk stratification with the risk chart from the European Society of Hypertension compared with SCORE in the general population. J Hypertens. 2009 Dec;27(12):2351-7. doi: 10.1097/HJH.0b013e328330e90a. PMID: 19915482.
- Naranjo A, Sokka T, Descalzo MA, Calvo-Alén J, Hørslev-Petersen K, Luukkainen RK, Combe B, Burmester GR, Devlin J, Ferraccioli G, Morelli A, Hoekstra M, Majdan M, Sadkiewicz S, Belmonte M, Holmqvist AC, Choy E, Tunc R, Dimic A, Bergman M, Toloza S, Pincus T; QUEST-RA Group. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10(2):R30. doi: 10.1186/ar2383. Epub 2008 Mar 6. PMID: 18325087; PMCID: PMC2453774.
- Serruys PW, Onuma Y, Garg S, Sarno G, van den Brand M, Kappetein AP, Van Dyck N, Mack M, Holmes D, Feldman T, Morice MC, Colombo A, Bass E, Leadley K, Dawkins KD, van Es GA, Morel MA, Mohr FW. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009 May;5(1):50-6. doi: 10.4244/eijv5i1a9. PMID: 19577983.