More Information
Submitted: February 19, 2023 | Approved: March 08, 2023 | Published: March 09, 2023
How to cite this article: Elgammal RM, Elsaiedy MA, Alamrosy MZ, Elsetiha ME, Almasry MM. Left ventricular assessment in patients with significant mitral incompetence: a multi-modality imaging study. J Cardiol Cardiovasc Med. 2023; 8: 012-020.
DOI: 10.29328/journal.jccm.1001148
Copyright License: © 2023 Elgammal RM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Primary mitral regurgitation; MR; CMR; 2D echo; 3D echo; GLS; Left ventricle
Left ventricular assessment in patients with significant mitral incompetence: a multi-modality imaging study
Reham Mostafa Elgammal* , Mona Adel Elsaiedy, Mahmoud Zki Alamrosy, Mohamed Elsaied Elsetiha and Magdy Mohamed Almasry
, Mona Adel Elsaiedy, Mahmoud Zki Alamrosy, Mohamed Elsaied Elsetiha and Magdy Mohamed Almasry
Department of Cardiovascular Medicine, Faculty of Medicine, Tanta University, Tanta, Egypt
*Address for Correspondence: Reham Mostafa Elgammal, Department of Cardiovascular Medicine, Faculty of Medicine, Tanta University, Tanta, Egypt, Email: [email protected]
Background: Detection of the deleterious effect of MR on LV is crucial in guiding the surgical decision.
Aim of the study: Comprehensive assessment of LV with significant primary MR using (2D, 3D echo and CMR).
Methods: 40 patients with significant MR have been recruited in a prospective study. Patients underwent 2D and 3D echo and CMR studies. LV volumes, function and GLS were calculated.
Results: End diastolic and systolic volumes were significantly larger when measured by CMR (all p < 0.001). EDV measures were strongly correlated with CMR and 3D echocardiography.
Conclusion: It’s important to identify early deleterious LV changes.
Primary Mitral Regurgitation (MR) includes abnormalities at any level of the Mitral Valve (MV) apparatus; mitral leaflets, chordae tendineae, papillary muscles and annulus [1]. Myxomatous degeneration, rheumatic disease, connective tissue disease and mitral annular calcification are the commonest causes of primary MR [2]. In the long run, untreated MR will lead to ventricular dilatation, irreversible LV dysfunction and ultimately heart failure [3]. ESC guidelines have identified the presence of symptoms in addition to an EF < 60% and Left Ventricle End-Systolic Dimension (LVESD) > 40 mm as a cutoff for MV repair/ replacement. These parameters usually don’t determine the actual transition to LV dysfunction [4]. In addition, unsatisfactory outcomes have been identified upon delaying MV surgery till meeting the cutoff parameters [5]. TTE is the first choice modality for the assessment of MR severity. It determines MR etiology and mechanism and quantifies its severity as well as the hemodynamic drawbacks on the left ventricle [6]. Assessment of LV systolic function is a cornerstone in the evaluation of mitral valve regurgitation guiding surgical intervention decisions [7]. Many conventional methods are available for assessment of LV Function using 2D echo i.e. linear Dimensions (M-mode) and modified Simpson Biplane Method which is currently recommended method of quantifying LV volume and systolic function [8]. Global Longitudinal Strain (GLS) measures the function of longitudinally orientated myofibers, which are most vulnerable to myocardial disease. Therefore, it can predict early subclinical myocardial dysfunction, particularly in patients with normal LVEF [7]. 3D echo has overcome many 2D echo limitations i.e. image foreshortening. Besides being superior in measuring LV volumes and function, it provides 3D speckle tracking echocardiography which overcomes (out-of-plane phenomenon) of 2D echo STE [9] CMR is currently a reference standard for assessment of LV function and mass with excellent temporal and spatial resolution [10]. CMR has a unique utility of myocardial tissue characterization, thus help in differentiating various myocardial pathologies [11]. Moreover, CMR feature tracking is a rising up technique which detects myocardial deformation and analyzes the global and segmental function. Therefore, it can help in the early detection of myocardial dysfunction [12]. In our study, we aimed assessment of left ventricle function in patients with significant mitral regurgitation using multi-modality imaging that includes; 2D echo, 3D echo and Cardiac Magnetic Resonance (CMR) for complementary evaluation.
Study design
A prospective observational study has been conducted in Aswan and Tanta heart centers. It included 40 consecutive patients who have been recruited from May 2019 to May 2021.
Inlusion criteria
We included all patients with significant (graded as moderate to severe or severe) primary mitral regurgitation were included within the study.
Exclusion criteria
We excluded patients with mild to moderate MR, patients with other valvular lesions that may affect left ventricle function i.e. severe aortic incompetence, Patients with atrial fibrillation or atrial flutter rhythm or any ventricular arrhythmia and patients with a contraindication to CMR i.e. claustrophobic were excluded. For all included patients the following were obtained: Full history taking (age, identification of risk factors i.e. diabetes mellitus, hypertension, obesity, smoking, current clinical complaint and related past history data were included), Full clinical examination (including heart rate, systolic and diastolic blood pressure) and 12 leads ECG were done.
All patients underwent 2D trans-thoracic echocardiography using a commercially available ultrasound machine (iE33, Philips Medical System, Andover, MA, USA). In the 2D study, End-Diastolic Dimension (EDD) and End-Systolic Dimensions (ESD) were measured in M mode. End-diastolic volume (EDV), End-Systolic Volume (ESV), Stroke Volume (SV) and LV Ejection Fraction (EF) were calculated by the biplane Simpson’s method. LV endocardium was traced contiguously from one side of the mitral annulus to the other side, including papillary muscles as part of the LV cavity. Apical four, two-chamber and long-axis views during three consecutive cardiac cycles were acquired in the left lateral decubitus position during a breath-hold. 2D speckle-tracking analysis was performed using vendor-independent speckle-tracking software (2D CPA, TomTec Imaging Systems, Unterschleissheim, Germany) and Global Longitudinal Strain (GLS) was calculated. After that, 3D full-volume datasets were acquired using an iE33 scanner (Philips Medical Systems) equipped with a fully sampled matrix-array transducer (X5 - 1) through an apical approach. Dataset of the entire left ventricle was acquired throughout at least 3 consecutive cardiac cycles with electrocardiographic gating during a single 5 – 7 s breath-hold. 3D volumetric measures of LV were measured using (4D LV Analysis, version 3.1.2, Tom Tec Imaging Systems, Unterschleissheim, Germany). 3D speckle-tracking swas obtained automatically by the use of the same software throughout the cardiac cycle. The software provided averaged longitudinal strain time curves from each segmental strain curve, from which peak global strains were determined. Finally, CMR imaging was performed for all patients using a 1.5 - T scanner (Siemens, Germany). Breath-hold ECG-gated steady-state free precession sequences in standard long-axis and multiple parallel short-axis slices were used for the assessment of end-systolic and end-diastolic LV volumes. LV stroke volume (end-diastolic LV volume – end-systolic LV volume) and the LVEF ([end-systolic volume/end-diastolic volume] × 100) were calculated from Cine short-axis slices. The antegrade LV stroke volume was obtained by phase contrast velocity mapping of the ascending aorta. Mitral RVol was calculated as the difference between the LV stroke volume and the antegrade LV stroke volume. Standard LGE-CMR sequences were used for assessment of myocardial fibrosis using a magnitude and phase-sensitive segmented inversion-recovery sequence approximately 10 min after intravenous gadolinium contrast administration (gadopentetate dimeglumine, 0.15 mmol/kg) LGE-CMR images were obtained in matching short- and long-axis planes. CMR Feature Tracking (FT) analysis CMR images were done using commercial feature tracking software (Segment software). LV endocardial and epicardial borders at the end-diastolic frame were manually drawn on a single frame and global longitudinal strain was determined by averaging the peak strain values.
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) Qualitative data were described using numbers and percentages. Kolmogorov-Smirnov test was used to verify the normality of distribution Quantitative data were described using range (minimum and maximum), mean, standard deviation, median and Interquartile Range (IQR). The significance of the obtained results was judged at the 5% level. The following test was used in the study: Chi-square test, Monte Carlo correction and Spearman coefficient test to correlate between two distributed abnormally quantitative variables and the p value which was considered significant if less than 0.05.
The mean age of the study population was 50.23 ± 14.31 with a range between 20 and 75 years. Out of the study population, 17 (43%) patients were females. It was noticed that the mean body mass index was 27.32 ± 5.12 kg/m2 with a range between 17.9 and 42 kg/m2. Most of the patients didn’t have a history of diabetes mellitus or hypertension and the majority were a non-smoker. The baseline demographic and clinical characteristics of the study population are summarized in Table 1.
| Table 1: The baseline demographic and clinical characteristics of the study population. | ||
| Primary MR (n = 40) | ||
| No. | % | |
| Gender | ||
| Male | 23 | 57.5 |
| Female | 17 | 42.5 |
| Age (years) | ||
| Min. – Max. | 20.0 – 74.0 | |
| Mean ± SD. | 50.23 ± 14.31 | |
| BMI (kg/m2) | ||
| Min. – Max. | 17.90 – 42.0 | |
| Mean ± SD. | 27.32 ± 5.12 | |
| DM | 2 | 5.0% |
| HTN | 4 | 10.0% |
| Smoker | 8 | 20.0% |
| BSA: Body Surface Area; BMI: Body Mass Index; DM: Diabetes Mellitus; HTN: Hypertension. | ||
The mean heart rate was 79.55 ± 14.32, while the mean systolic blood pressure was 119.68 ± 16.75 and the mean diastolic blood pressure was 74.25 ± 11.47. The basic clinical examination parameters of the study population are summarized in Table 2.
| Table 2: The basic clinical examination parameters of the study population. | |
| Clinical parameters | Primary MR (n = 40) |
| HR | |
| Min. – Max. | 56.0 – 110.0 |
| Mean ± SD. | 79.55 ± 14.32 |
| SBP | |
| Min. – Max. | 77.0 – 150.0 |
| Mean ± SD. | 119.68 ± 16.75 |
| DBP | |
| Min. – Max. | 50.0 – 90.0 |
| Mean ± SD. | 74.25 ± 11.47 |
| HR: Heart Rate; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure. | |
Among patients of the study, 2 out of 40 cases presented typical chest pain and accounted for 5% of cases. Heart failure symptoms i.e. dyspnea were the main presentation in 19/40 and accounted for 47.5%, while the rest 19/40 cases (47.5%) were completely asymptomatic. The main presenting symptoms of the study population are summarized in Table 3.
| Table 3: The main presenting symptoms of the study population. | ||
| Presentation | Primary MR (n = 40) | |
| No. | % | |
| HF | 19 | 47.5 |
| T.C.P | 2 | 5.0 |
| Asymptomatic | 19 | 47.5 |
| HF: Heart Failure; TCP: Typical Chest Pain. | ||
Mitral valve prolapse with and without MAD was the commonest cause of MR, it was detected in 20/40 cases and accounted for 50% of the causes. Rheumatic heart disease came in second place and was found in 8/40 cases, representing 20% of primary MR cases. The rest of the causes were; flail posterior mitral leaflet 5/40 cases, degenerative mitral valve 5/40, each of them accounted for 12.5%, perforation of the anterior mitral leaflet by infective endocarditis was detected in one case and a single case showed flail anterior mitral leaflet, each represented 2.5%. The etiology of primary mitral regurgitation is illustrated in Table 4.
| Table 4: Etiology of mitral regurgitation of the study population. | ||
| Etiology | Primary MR (n = 40) | |
| No. | % | |
| Degenerative | 5 | 12.5 |
| Flail AML | 1 | 2.5 |
| Flail PML | 5 | 12.5 |
| MAD, MVP | 5 | 12.5 |
| MVP | 15 | 37.5 |
| MV perforation | 1 | 2.5 |
| RHD | 8 | 20.0 |
| AML: Anterior Mitral Leaflet; PML: Posterior Mitral Leaflet; MVP: Mitral Valve Prolapse; RHD: Rheumatic Heart Disease. | ||
Based on 2D echocardiographic findings; it was noticed that the study population had mean end-diastolic dimensions of 5.55 ± 0.83 while mean end-systolic dimensions were 3.60 ± 0.78. Biplane Simpson’s volumetric method showed mean end-diastolic volume and end-systolic volume 166.25 ± 33.71 and 61.58 ± 17.09 respectively. The mean stroke volume was 112.40 ± 17.69 and the mean ejection fraction was 62.53 ± 7.34. The mean global longitudinal strain was -21.64 ± 4.03 .3D volumetric method showed mean EDV and mean ESV 180.48 ± 32.28 and 68.13 ± 26.59 respectively. The mean stroke volume was 112.40 ± 17.69 and the mean ejection fraction was 62.52 ± 8.70. The mean global longitudinal strain was -23.50 ± 9.92. The results of 2D and 3D echo volumetric measurements are summarized in Table 5.
| Table 5: 2D and 3D echocardiographic volumetric measurement of the study population. | ||
| Echo parameters | Primary MR (n = 40) | |
| EDD | 2d | |
| Min. – Max. | 4.30 – 6.90 | |
| Mean ± SD. | 5.55 ± 0.83 | |
| ESD | 2d | |
| Min. – Max. | 2.30 – 5.80 | |
| Mean ± SD. | 3.60 ± 0.78 | |
| EDV | 2d | |
| Min. – Max. | 105.0 – 226.0 | |
| Mean ± SD. | 166.25 ± 33.71 | |
| 3d | ||
| Min. – Max. | 128.0 – 254.0 | |
| Mean ± SD. | 180.48 ± 32.28 | |
| ESV | 2d | |
| Min. – Max. | 39.0 – 105.0 | |
| Mean ± SD. | 61.58 ± 17.09 | |
| 3d | ||
| Min. – Max. | 31.0 – 137.0 | |
| Mean ± SD. | 68.13 ± 26.59 | |
| 2d | ||
| Min. – Max. | 54.0 – 155.0 | |
| Mean ± SD. | 104.68 ± 26.14 | |
| 3d | ||
| Min. – Max. | 70.0 – 146.0 | |
| Mean ± SD. | 112.40 ± 17.69 | |
| EF | 2d | |
| Min. – Max. | 46.0 – 77.0 | |
| Mean ± SD. | 62.53 ± 7.34 | |
| 3d | ||
| Min. – Max. | 45.0 – 78.0 | |
| Mean ± SD. | 62.52 ± 8.70 | |
| GLS | 2d | |
| Min. – Max. | -29.20 – -11.10 | |
| Mean ± SD. | -21.64 ± 4.03 | |
| 3d | ||
| Min. – Max. | -32.60 – 30.80 | |
| Mean ± SD. | -23.50 ± 9.92 | |
| EDD: End-Diastolic Dimension; ESD: End-Systolic Dimension; EDV: End-Diastolic Volume; ESV: End-Systolic Volume; SV: Stroke Volume; EF: Ejection Fraction; GLS: Global Longitudinal Strain. | ||
By CMR, it was noticed that the mean of EDV and ESV were 220.63 ± 53.15 and 87.60 ± 35.04 respectively. Mean stroke volume was 132.33 ± 25.52 and the mean ejection fraction was 61.0 ± 7.90. The mean global longitudinal strain mean was 20.47 ± 3.58. The mean mitral regurgitant volume measured by CMR was 58.28 ± 19.87, while the mean regurgitation fraction was 43.80 ± 8.86. The results of CMR volumetric measurements are summarized in Table 6.
| Table 6: MRI volumetric measurements and remolding parameters. | |
| MRI parameters | Primary MR (n = 40) |
| EDV | |
| Min. – Max. | 135.0 – 395.0 |
| Mean ± SD. | 220.63 ± 53.15 |
| ESV | |
| Min. – Max. | 39.0 – 211.0 |
| Mean ± SD. | 87.60 ± 35.04 |
| SV | |
| Min. – Max. | 81.0 – 189.0 |
| Mean ± SD. | 132.33 ± 25.52 |
| EF% | |
| Min. – Max. | 41.0 – 78.0 |
| Mean ± SD. | 61.0 ± 7.90 |
| AO | |
| Min. – Max. | 43.0 – 104.0 |
| Mean ± SD. | 74.0 ± 14.13 |
| RV | |
| Min. – Max. | 29.0 – 113.0 |
| Mean ± SD. | 58.28 ± 19.87 |
| RF% | |
| Min. – Max. | 35.0 – 69.0 |
| Mean ± SD. | 43.80 ± 8.86 |
| GLS | |
| Min. – Max. | -27.90 – -12.20 |
| Mean ± SD. | -20.47 ± 3.58 |
| EDV: End-Diastolic Volume; ESV: End Systolic Volume; SV: Stroke Volume; EF: Ejection Fraction; GLS: Global Longitudinal Strain; AO: Aortic Forward Flow; RV: Regurgitant Volume; RF: Regurgitant Fraction. | |
The majority of patients 26/40 showed no fibrosis at all, they accounted for 65% of cases. While 11/40 cases showed a non-specific pattern of fibrosis (i.e. insertion point fibrosis) and they represented 27.5% of cases. Only 3 out of 40 cases showed a specific pattern (non-territorial focal sub-endocardial), they accounted for 7.5% of the study population. Pattern of myocardial fibrosis detected by CMR is demonstrated in Table 7.
| Table 7: Patterns of myocardial fibrosis detected by cardiac MRI. | ||
| Fibrosis | Primary MR (n = 40) | |
| No. | % | |
| None | 26 | 65.0 |
| None specific | 11 | 27.5 |
| Specific | 3 | 7.5 |
In primary mitral regurgitation patients, EDV was significantly larger when measured by CMR (mean EDV = 220.63) as compared to 3D echo (mean EDV = 180.43) and 2D echo (mean EDV = 166.25) which significantly underestimated EDV (all p < 0.001). However, the volumetric measures of 2D End-Diastolic Volume (EDV) were strongly correlated with CMR (the gold standard for quantification of left ventricular volumes and function) and 3D echocardiography (r = 0.88, p < 0.001). In the same way, ESV was significantly larger when measured by CMR (mean ESV = 87.6) as compared to 3D echo (mean ESV = 68.13) and 2D echo (mean ESV = 61.58) which significantly underestimated ESV (all p < 0.001). However, 2D echo end-systolic volume (ESV) measures were strongly correlated with CMR and 3D echo (r = 0.7 and 0.63 respectively, p < 0.001). 3D echo ESV measures were moderately correlated with CMR (r = 0.5, p < 0.001) .2D echo significantly underestimated stroke volume (mean SV = 104.68) as compared to 3D echo (mean SV = 112.4) and CMR (mean SV = 132.33), (all p < 0.001). On the other side 2D echo measures of stroke volume were strongly correlated with 3D echo measures (r = 0.8, p < 0.001), however, both echo modalities were moderately correlated with CMR (all r = 0.5, p < 0.001).
Regarding Ejection fraction, 2D echo measured mean EF = 62.52 while 3D echo means EF was 62.53 and CMR mean EF was 61.0 (p > 0.05) .2D echo measures of ejection fraction (EF) showed a strong correlation with 3D echo measures (r = 0. 8, p < 0.001), however, both modalities were weekly correlated with CMR (all r < 0.5, p > 0.05).
GLS values measured by 2D echo showed mean GLS = -21.46, while mean GLS measured by 3D echo was -23.5 and by CMR = -20.47.GLS values measured by 2D and CMR were moderately correlated (r = 0.5, p < 0.001), while 3D echo measures were weakly correlated with 2D echo and CMR measures (all r < 0.5, p = 0.012 and 0.064 respectively).
The correlation between the three imaging modalities measurements is summarized in Table 8.
| Table 8: Correlation between three imaging modalities in measuring different parameters. | ||||||
| 2d | 3d | MRI | Coefficient | |||
| 2d vs. 3d | 2d vs. MRI | 3d vs. MRI | ||||
| EDV | 166.25 | 180.48 | 220.63 | r = 0.809 p < 0.001* |
r = 0.717 p < 0.001* |
r = 0.887 p < 0.001* |
| ESV | 61.58 | 68.13 | 87.60 | rs = 0.727 p< 0.001* |
rs = 0.639 p < 0.001* |
rs = 0.517 p = 0.001* |
| SV | 104.68 | 112.40 | 132.33 | rs = 0.886 p <0.001* |
rs = 0.509 p=0.001* |
rs = 0.558 p < 0.001* |
| EF | 62.53 | 62.52 | 61.0 | rs = 0.863 p < 0.001* |
rs = 0.291 p = 0.068 |
rs = 0.306 p = 0.055 |
| GLS | -21.64 | -23.50 | -20.47 | rs = 0.393 p = 0.012* |
rs = 0.564 p < 0.001* |
rs = 0.296 p = 0.064 |
| EDV: End-Diastolic Volume; ESV: End-Systolic Volume; SV: Stroke Volume; EF: Ejection Fraction; GLS: Global Longitudinal Strain; r = Correlation Coefficient. | ||||||
Case 1 (myxomatous mitral valve disease with flail posterior mitral leaflet)
62 years old male, not known to be diabetic or hypertensive or a smoker presented with dyspnea on minimal exertion (NYHA class III). 2D echocardiography revealed flail posterior mitral leaflet with severe mitral regurgitation resulting in mild dilatation of LV, mildly impaired systolic function, EF = 56% and mildly impaired GLS = -17.2%. 3D echo revealed the same data with EF = 55%, GLS = -19.4%. CMR showed mildly dilated LV with mildly impaired LV systolic function, EF = 57%, GLS = -17.7%. No fibrosis was detected at LGE (Figures 1-5).
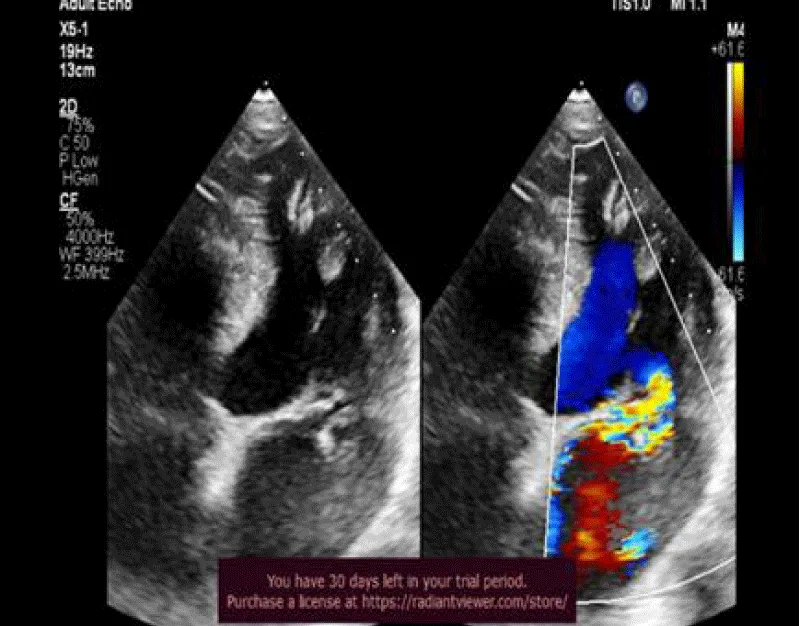
Figure 1: 2DTTE, 4 chamber view revealed myxomatous mitral valve disease with flail posterior mitral leaflet with severe MR, eccentric jet.
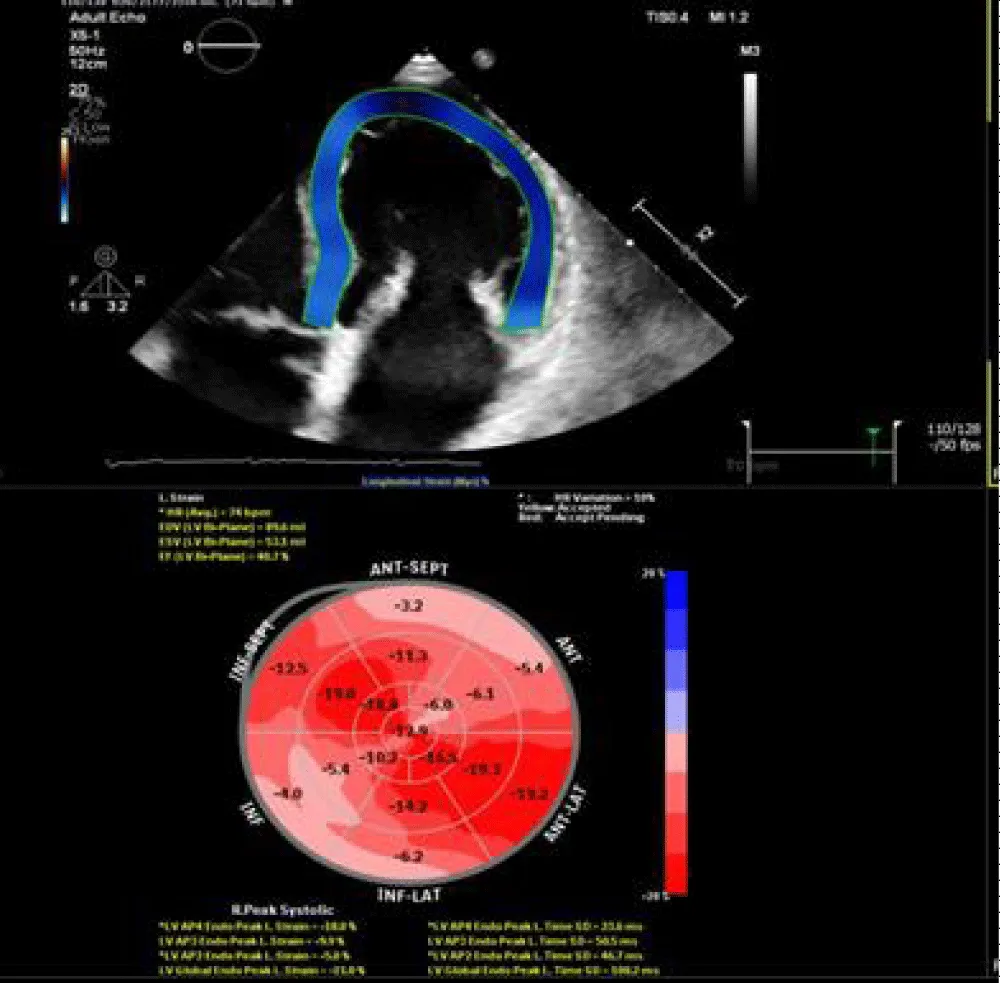
Figure 2: 2D speckle tracking of left ventricle revealed mildly impaired GLS = -17.2%.
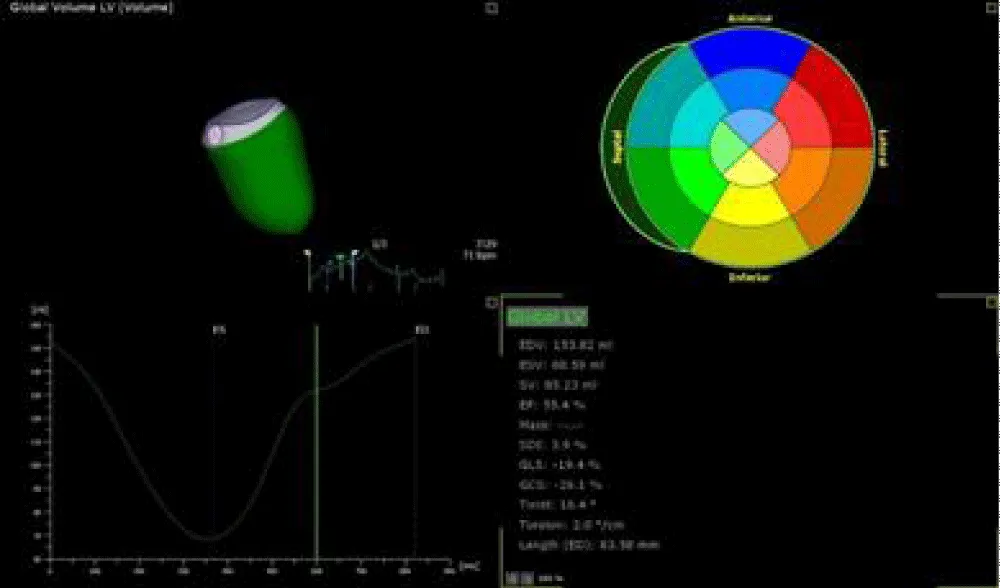
Figure 3: 3D TEE revealed mildly dilated LV volumes with GLS = -19.4%.
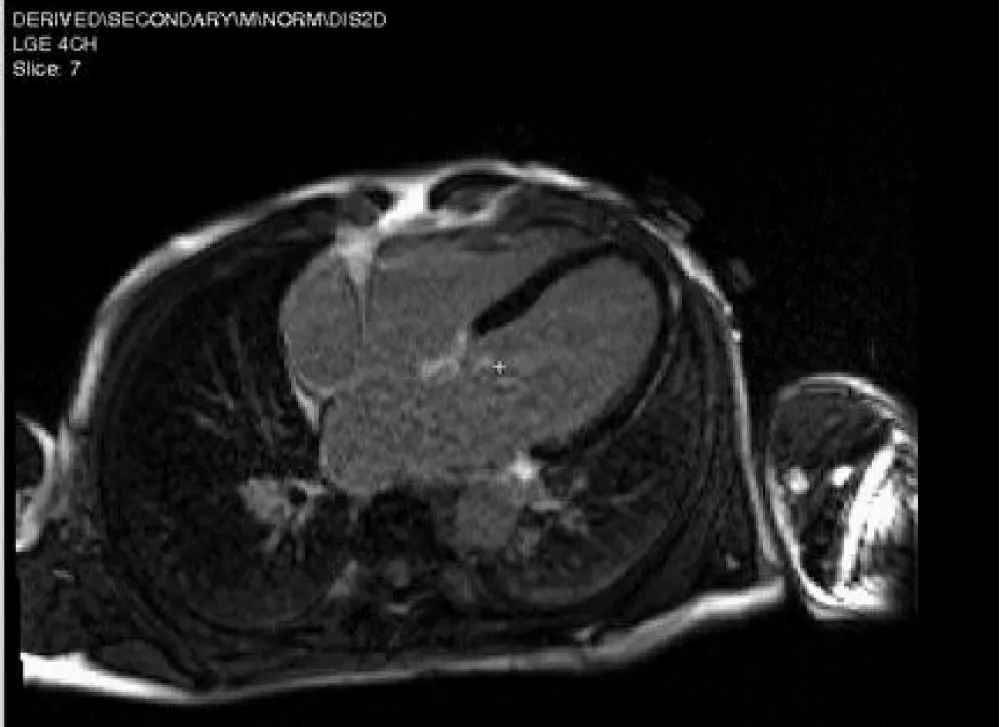
Figure 4: CMR-LGE 4 chamber view revealed no fibrosis.
Figure 5: Feature tracking of LV revealed mildly impaired GLS = -17.7%. Primary mitral regurgitation.
Case 2 mitral annulus disjunction (MAD) with mitral valve prolapse
25 years old male, presented by palpation. 2D echocardiography revealed MAD with mitral valve prolapse causing moderate to severe mitral regurgitation. Average LV volumes were noted with preserved systolic function, EF = 62 % and GLS = -19.7%. 3D echo revealed average LV volumes with mildly impaired, EF = 52% and mildly impaired GLS = -17.2%. CMR showed average LV with preserved LV systolic function, EF = 64% and impaired GLS = -13.1%. Nonterritorial subendocardial fibrosis was noted affecting the mid-segment of inferolateral and anterolateral walls (Figures 6-11).
Figure 6: 2D TTE revealed bi-leaflet MVP causing moderate to severe MR.
Figure 7: 2D TTE revealed MAD(systolic displacement distance =4.1 mm ,arrow).
Figure 8: 2D speckle tracking of LV revealed GLS =-19.7%.
Figure 9: 3D TTE revealed average LV volumes with GLS =-17.2%.
Figure 10: CMR -LGE, 3 chamber view revealed nonterritorial sub-endocardial fibrosis of lateral wall (arrows).
Figure 11: Feature tracking revealed impaired GLS = -13.1%.
Mitral valve regurgitation is a major cause of morbidity and mortality across the world [13]. From a pathophysiology perspective, MR implicates a pure volume overload on the LV and subsequently decreases forward stroke volume. In the early stages, eccentric LV hypertrophy compensates for low forward stroke volume. However, eventually, volume overload causes LV dysfunction [14]. The main issue in MR is that the favorable loading conditions made by a less resistant pathway (left atrium) often mask LV dysfunction. The second pathway aids in reducing the afterload, while the volume overload itself increases preload leading to supernormal ejection fraction [14]. Currently, the best markers of functional deterioration are the development of symptoms in addition to echocardiographic parameters of systolic failure (EF < 60% and LVESD = 4.5) [15]. Since the comprehensive evaluation of LV is crucial in the surgical management of MR, we used multimodality imaging tools in order to fully evaluate LV volumes and function, in addition to detection of early subclinical dysfunction and remolding using GLS.
Our study revealed that in primary mitral regurgitation, EDV was significantly larger when measured by CMR (mean EDV = 220.63) compared to 3D echo (mean EDV = 180.43) and 2D echo (mean EDV = 166.25) which significantly underestimated EDV (all p < 0.001). In the same way, ESV was significantly larger when measured by CMR (mean ESV = 87.6) as compared to 3D echo (mean ESV = 68.13) and 2D echo (mean ESV = 61.58) which significantly underestimated ESV (all p < 0.001). However, 2D echo end-systolic volume (ESV) measures were strongly correlated with CMR and 3D echo (r = 0.7 and 0.63 respectively, p < 0.001) .3D echo ESV measures were moderately correlated with CMR (r = 0.5, p < 0.001).
2D echo significantly underestimated stroke volume (mean SV = 104.68) as compared to 3D echo (mean SV = 112.4) and CMR (mean SV =132.33), (all p < 0.001). On the other side 2D echo measures of stroke volume were strongly correlated with 3D echo measures (r = 0.8, p < 0.001), however, both echo modalities were moderately correlated with CMR (all r = 0.5, p < 0.001). Regarding Ejection fraction, 2D echo measured mean EF = 62.52 while 3D echo means EF was 62.53 and CMR mean EF was 61.0 (p > 0.05). 2D echo measures of Ejection Fraction (EF) showed a strong correlation with 3D echo measures (r = 0. 8, p < 0.001), however, both modalities were weekly correlated with CMR (all r < 0.5, p > 0.05). GLS values measured by 2D echo showed mean GLS = -21.46, while mean GLS measured by 3D echo was -23.5 and by CMR = -20.47.GLS values measured by 2D and CMR were moderately correlated (r = 0.5, p < 0.001), while 3D echo measures were weakly correlated with 2D echo and CMR measures (all r < 0.5, p = 0.012 and 0.064 respectively.
These results helped us in figuring out the utility of different used imaging modalities in the assessment of MR impact on LV. The results came in concordance with many previous types of research that were dedicated to the assessment of LV volumes and function in primary MR.
Firstly, the underestimation of 2D volumetric measure-ments in comparison with 3D echo and gold standard CMR was expected and has been explained by the poor image quality, apical foreshortening and geometrical assumptions [16]. This came in concordance with Levy F, et al. described 53 patients with at least mild primary isolated MR, who underwent comprehensive 3D transthoracic echo and CMR studies within 24 h. Compared with CMR, which is the gold standard for cardiac chamber quantification, LV volumes calculated from 3D TTE showed significantly smaller bias and lower intra- and interobserver variability than 2D TTE, EDV obtained by the different methods (CMR and 3D echo) bias = −12 ± 22 mL, with a significant correlation (r = 0.93; p < 0.0001). ESV obtained by the different methods showed the same pattern of underestimation by 3D echo with also a small bias (bias = −6 ± 20 mL). LV ejection fraction was similar between CMR and 3D echo with a significant correlation between the two measurements (r = 0.81; p < 0.0001). Despite the bias in the present study being larger than that of Levy et.al (bias for EDV = 40.15 ml and ESV = 19.32 ml), a very strong correlation, (r = 0.8) between 3D echocardiography and CMR was found in our study as he found out [17].
Also, Van De Heyning CM, et al. prospectively included 38 patients with at least moderate primary mitral regurgitation, a left ventricular ejection fraction ≥ 60% and a left ventricular end-systolic diameter ≤ 45 mm. All patients were scheduled for 2D TTE and CMR. LV dimensions and volumes. They found that LV volumes were significantly underestimated by 2D TTE in patients with moderate to severe primary MR in comparison with CMR. It showed a mean difference of 28 ml for the LV EDV and 20 ml for LV-ESV. As regard LV EF in his study both the Teichholz formula and modified Simpson’s method by 2D TTE seemed to overestimate LVEF in comparison to CMR [18].
Secondly, 20% of our patients with severe MR and preserved LV function (LVEF ≥ 60%) by 2D TTE Simpson’s method had a mild decreased LVEF (50% - 59%) by CMR. These observations are in line with Van De Heyning CM, et al. study in which 33% of his patients with severe MR and preserved LV function (LVEF ≥ 60%) by 2D TTE Simpson’s method had a mild decreased LVEF (50% - 59%) by CMR and suggested that more accurate assessment of LVEF by CMR might be indicated in asymptomatic severe MR to determine optimal timing for surgery [18]. In patients who do not meet Class I recommendation for surgery, it remains questionable, whether an early surgery versus watchful waiting approach is superior. Recognizing that delaying surgery until the onset of ventricular dysfunction with left ventricular ejection fraction (LVEF) < 60% or LVESD > 40 mm, may be associated with an outcome penalty [5]. Hence, more sensitive and accurate imaging may provide more data on the optimum timing of surgery. In our study, GLS has been used for the detection of LV subclinical dysfunction. 2021 ESC guidelines declared that despite GLS showing promise as a reliable marker for the detection of subclinical LV dysfunction in Primary MR, further investigation is required to determine a predictive GLS cut‐off that demonstrates both reproducibility and efficacy [15].
Another literature agrees there is a significant correlation between GLS and LVEF presented by Mascle S, et al. who illustrated that impaired preoperative GLS was negatively correlated with postoperative LVEF. She recruited 88 patients with severe degenerative MR who underwent rest echocardiography before and 6 ± 1 months after mitral valve surgery. This study demonstrated the additive and independent predictive value of preoperative GLS for predicting postoperative LV dysfunction [19]. Alashi A. Also confirmed the same results in his study in 2016 that included 48 asymptomatic patients with ≥ 3+ mitral regurgitation and preserved LV EF who underwent mitral valve surgery [20]. A remaining question is, which modality would be better for measurement of GLS in primary MR? Hans-Joachim Nesser, et al. had nearly the same results as our study by obtaining GLS using 2D TTE, 3D TTE, and CMR in 43 patients with a wide range of LV sizes and functions. 2D-STE strongly correlated with CMR (r : 0.72 – 0.88), however, it underestimated LV volumes with relatively large biases (10 mL – 30 mL). The 3D-STE measurements showed a higher correlation with CMR (0.87 – 0.92), and importantly smaller biases (1 mL – 16 mL) [21]. This showed a strong agreement with our study.
At last, our study revealed 35% of the patients had a different pattern of patchy fibrosis. although the study didn’t focus on the prognostic value of the fibrosis, it remains one of the most important factors in arrhythmic events of sudden cardiac death [15]. Danai Kitkungvan, et.al enrolled 356 primary MR patients (177 MVP and 179 non-MVP). LV fibrosis was more prevalent in the MVP group than in the non-MVP group (36.7% vs. 6.7%). During follow-up, MVP patients with LV fibrosis had the highest event rate for arrhythmic events [22]. Another two previous studies with smaller sample sizes of patients with chronic, asymptomatic moderate, or severe primary MR have also illustrated a high prevalence of LV fibrosis. The first one was made by Edwards NC, et.al who recruited 35 patients with asymptomatic moderate or severe primary degenerative MR. His patients were compared with age and sex controls and underwent cardiopulmonary exercise testing, echocardiography, and cardiac MRI. Longitudinal and circumferential myocardial deformation was reduced with MR in patients with EF (67% ± 10%). Myocardial extracellular volume was increased (0.32 ± 0.07 versus 0.25 ± 0.02, p < 0.01) and was associated with increased indexed ESV (r = 0.62, p < 0.01), indexed left atrial volume (r = 0.41, p < 0.05). He concluded that LV-indexed ESV and left atrial volume were independent predictors of extracellular volume (r (2) = 0.42, p < 0.01) [23]. The second study was made by Van De Heyning CM, et al. who showed that out of his 39, 12 (31%) had late contrast uptake of the LV wall. LGE CMR showed an infarct pattern in three patients, a pattern of mid-wall fibrosis in seven patients and two patients had a combined pattern. Patients with fibrosis on CMR had significantly higher LV diameters (LV end-systolic diameter 39 ± 4 vs. 34 ± 5 mm, p = 0·002; LV end-diastolic diameter 57 ± 5 vs. 50 ± 5 mm, p = 0·001) [24].
Mitral valve regurgitation (MR) is a major source of morbidity and death worldwide. The left ventricle is playing an important role in the prognosis and intervention decision in MR, therefor comprehensive evaluation of LV using different imaging modalities is recommended. We concluded that CMR followed by 3D echo was more sensitive in the assessment of LV EDV and ESV and were strongly correlated, while 2D echo End-Systolic Volume (ESV) measures were strongly correlated with CMR and 3D echo. we also illustrated the parameters for the detection of myocardial fibrosis as GLS may be helpful in the detection of sub-clinical LV dysfunction, particularly in those with apparently normal LV ejection fraction.
Limitations of the study
Small sample size may affect the results, lack of postoperative follow-up, no definite cutoff for GLS value in primary MR and lack of quantitative measures of fibrosis detected by LGE – CMR.
Ethical consideration
Informed consent was obtained from the study population with consideration of their privacy and confidentiality. The research was approved by the Tanta university research ethics committee on 5/2019.
- Harb SC, Griffin BP. Mitral Valve Disease: a Comprehensive Review. Curr Cardiol Rep. 2017 Aug;19(8):73. doi: 10.1007/s11886-017-0883-5. PMID: 28688022.
- Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017 Apr;30(4):303-371. doi: 10.1016/j.echo.2017.01.007. Epub 2017 Mar 14. PMID: 28314623.
- Podlesnikar T, Delgado V, Bax JJ. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int J Cardiovasc Imaging. 2018 Jan;34(1):97-112. doi: 10.1007/s10554-017-1195-y. Epub 2017 Jun 22. PMID: 28642994; PMCID: PMC5797565.
- Quintana E, Suri RM, Thalji NM, Daly RC, Dearani JA, Burkhart HM, Li Z, Enriquez-Sarano M, Schaff HV. Left ventricular dysfunction after mitral valve repair--the fallacy of "normal" preoperative myocardial function. J Thorac Cardiovasc Surg. 2014 Dec;148(6):2752-60. doi: 10.1016/j.jtcvs.2014.07.029. Epub 2014 Jul 31. PMID: 25173130.
- Enriquez-Sarano M, Suri RM, Clavel MA, Mantovani F, Michelena HI, Pislaru S, Mahoney DW, Schaff HV. Is there an outcome penalty linked to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc Surg. 2015 Jul;150(1):50-8. doi: 10.1016/j.jtcvs.2015.04.009. Epub 2015 Apr 9. PMID: 25986494.
- Chew PG, Bounford K, Plein S, Schlosshan D, Greenwood JP. Multimodality imaging for the quantitative assessment of mitral regurgitation. Quant Imaging Med Surg. 2018 Apr;8(3):342-359. doi: 10.21037/qims.2018.04.01. PMID: 29774187; PMCID: PMC5941213.
- Luis SA, Chan J, Pellikka PA. Echocardiographic Assessment of Left Ventricular Systolic Function: An Overview of Contemporary Techniques, Including Speckle-Tracking Echocardiography. Mayo Clin Proc. 2019 Jan;94(1):125-138. doi: 10.1016/j.mayocp.2018.07.017. PMID: 30611439.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015 Jan;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003. PMID: 25559473. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440-63. doi: 10.1016/j.echo.2005.10.005. PMID: 16376782.
- Seo Y, Ishizu T, Atsumi A, Kawamura R, Aonuma K. Three-dimensional speckle tracking echocardiography. Circ J. 2014;78(6):1290-301. doi: 10.1253/circj.cj-14-0360. Epub 2014 Apr 28. PMID: 24770358.
- Zorzi A, Susana A, De Lazzari M, Migliore F, Vescovo G, Scarpa D, Baritussio A, Tarantini G, Cacciavillani L, Giorgi B, Basso C, Iliceto S, Bucciarelli Ducci C, Corrado D, Perazzolo Marra M. Diagnostic value and prognostic implications of early cardiac magnetic resonance in survivors of out-of-hospital cardiac arrest. Heart Rhythm. 2018 Jul;15(7):1031-1041. doi: 10.1016/j.hrthm.2018.02.033. Epub 2018 Mar 15. PMID: 29550522.
- Doltra A, Amundsen BH, Gebker R, Fleck E, Kelle S. Emerging concepts for myocardial late gadolinium enhancement MRI. Curr Cardiol Rev. 2013 Aug;9(3):185-90. doi: 10.2174/1573403x113099990030. PMID: 23909638; PMCID: PMC3780343.
- Schuster A, Morton G, Hussain ST, Jogiya R, Kutty S, Asrress KN, Makowski MR, Bigalke B, Perera D, Beerbaum P, Nagel E. The intra-observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol. 2013 Feb;82(2):296-301. doi: 10.1016/j.ejrad.2012.11.012. Epub 2012 Dec 12. PMID: 23246014.
- Levine RA, Hagége AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez-Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Mérot J, Messas E, Milan DP, Neri T, Norris RA, Peal D, Perrocheau M, Probst V, Pucéat M, Rosenthal N, Solis J, Schott JJ, Schwammenthal E, Slaugenhaupt SA, Song JK, Yacoub MH; Leducq Mitral Transatlantic Network. Mitral valve disease--morphology and mechanisms. Nat Rev Cardiol. 2015 Dec;12(12):689-710. doi: 10.1038/nrcardio.2015.161. Epub 2015 Oct 20. PMID: 26483167; PMCID: PMC4804623.
- Salihi S, Güden M. Durability of mitral valve repair: A single center experience. Turk Gogus Kalp Damar Cerrahisi Derg. 2019 Oct 23;27(4):459-468. doi: 10.5606/tgkdc.dergisi.2019.18165. PMID: 32082910; PMCID: PMC7018146.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021 Oct 22;60(4):727-800. doi: 10.1093/ejcts/ezab389. Erratum in: Eur J Cardiothorac Surg. 2022 Mar 24;61(4):964. Erratum in: Eur J Cardiothorac Surg. 2022 Jun 15;62(1): PMID: 34453161.
- Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA; American Society of Echocardiography; European Association of Echocardiography. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012 Jan;25(1):3-46. doi: 10.1016/j.echo.2011.11.010. PMID: 22183020.
- Levy F, Marechaux S, Iacuzio L, Schouver ED, Castel AL, Toledano M, Rusek S, Dor V, Tribouilloy C, Dreyfus G. Quantitative assessment of primary mitral regurgitation using left ventricular volumes obtained with new automated three-dimensional transthoracic echocardiographic software: A comparison with 3-Tesla cardiac magnetic resonance. Arch Cardiovasc Dis. 2018 Aug-Sep;111(8-9):507-517. doi: 10.1016/j.acvd.2017.10.008. Epub 2018 Mar 31. PMID: 29610031.
- Van De Heyning CM, Magne J, Piérard LA, Bruyère PJ, Davin L, De Maeyer C, Paelinck BP, Vrints CJ, Lancellotti P. Assessment of left ventricular volumes and primary mitral regurgitation severity by 2D echocardiography and cardiovascular magnetic resonance. Cardiovasc Ultrasound. 2013 Dec 27;11:46. doi: 10.1186/1476-7120-11-46. PMID: 24373138; PMCID: PMC3880971.
- Mascle S, Schnell F, Thebault C, Corbineau H, Laurent M, Hamonic S, Veillard D, Mabo P, Leguerrier A, Donal E. Predictive value of global longitudinal strain in a surgical population of organic mitral regurgitation. J Am Soc Echocardiogr. 2012 Jul;25(7):766-72. doi: 10.1016/j.echo.2012.04.009. Epub 2012 May 19. PMID: 22609096.
- Alashi A, Mentias A, Patel K, Gillinov AM, Sabik JF, Popović ZB, Mihaljevic T, Suri RM, Rodriguez LL, Svensson LG, Griffin BP, Desai MY. Synergistic Utility of Brain Natriuretic Peptide and Left Ventricular Global Longitudinal Strain in Asymptomatic Patients With Significant Primary Mitral Regurgitation and Preserved Systolic Function Undergoing Mitral Valve Surgery. Circ Cardiovasc Imaging. 2016 Jul;9(7):e004451. doi: 10.1161/CIRCIMAGING.115.004451. PMID: 27342145.
- Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, Sugeng L, Lang RM. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI. Eur Heart J. 2009 Jul;30(13):1565-73. doi: 10.1093/eurheartj/ehp187. Epub 2009 May 29. PMID: 19482868.
- Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, Little SH, Quinones MA, Lawrie GM, Zoghbi WA, Shah DJ. Myocardial Fibrosis in Patients With Primary Mitral Regurgitation With and Without Prolapse. J Am Coll Cardiol. 2018 Aug 21;72(8):823-834. doi: 10.1016/j.jacc.2018.06.048. PMID: 30115220.
- Edwards NC, Moody WE, Yuan M, Weale P, Neal D, Townend JN, Steeds RP. Quantification of left ventricular interstitial fibrosis in asymptomatic chronic primary degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2014 Nov;7(6):946-53. doi: 10.1161/CIRCIMAGING.114.002397. Epub 2014 Aug 19. PMID: 25140067.
- Van De Heyning CM, Magne J, Piérard LA, Bruyère PJ, Davin L, De Maeyer C, Paelinck BP, Vrints CJ, Lancellotti P. Late gadolinium enhancement CMR in primary mitral regurgitation. Eur J Clin Invest. 2014 Sep;44(9):840-7. doi: 10.1111/eci.12306. PMID: 25066426.