More Information
Submitted: May 03, 2023 | Approved: May 12, 2023 | Published: May 15, 2023
How to cite this article: Jourdain P, Picard F, Girerd N, Lemieux H, Barritault F, et al. Security and performance of remote patient monitoring for chronic heart failure with Satelia® Cardio: First results from real-world use. J Cardiol Cardiovasc Med. 2023; 8: 042-050.
DOI: 10.29328/journal.jccm.1001152
Copyright License: © 2023 Jourdain P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chronic heart failure; Telemedicine; Telehealth; Remote patient monitoring; Real-world evidence; Clinical algorithm
Security and performance of remote patient monitoring for chronic heart failure with Satelia® Cardio: First results from real-world use
Patrick Jourdain1*, F Picard2, N Girerd3, H Lemieux4, F Barritault5, MF Seronde6, JP Labarre7, N Pages2, C Bedel8, L Betito8, S Nisse-Durgeat9 and B Diebold10
1Cardiology Department, Paris-Saclay University, Le Kremlin Bicêtre, France
2Cardiology Department, Haut Leveque Hospital, Bordeaux, France
3Cardiology Department, Nancy, France
4Cardiology Department, Esquirol Saint Hilaire Clinic, Agen, France
5Cardiology Department, GCS Cardiology, Bayonne Hospital, Bayonne, France
6Cardiology Department, University of Besançon, Besancon, France
7Cardiology Department, Pont de Chaume Clinic, Montauban, France
8NP Medical, Bordeaux, France
9Cardiology Department, Cochin Hospital, Paris, France
*Address for Correspondence: Patrick Jourdain, Cardiology Department, Paris-Saclay University, Le Kremlin Bicêtre, France, Email: [email protected]
Background: Since 2019, remote patient monitoring (RPM) for patients with chronic heart failure (CHF) has been supported by the European Society of Cardiology. However, real-world data on the use of such solutions has been limited and not primarily based on patient-reported outcomes. The aim of this study was to describe the Satelia® Cardio solution in France within the French ETAPES funding program and assess the security and performance of its clinical algorithm.
Methods: A retrospective observational study was conducted on CHF patients monitored by RPM through Satelia® Cardio. From September 1, 2018, to June 30, 2020, patients were included if they had completed over six months of follow-up. The risk of a possible CHF decompensation was categorized by the system in three levels: green, orange and red. The algorithm security and performance were assessed through the negative predictive value (NPV) of the prediction of hospitalization of a patient within seven days.
Results: In total, 331 patients were included in this study with 36,682 patient self-administered questionnaires answered. Patients were mostly males (70.4%) and had a mean age of 68.1 years. The mean left ventricular ejection fraction (LVEF) was 35.4% (± 12.3) and 73.3% of patients had a LVEF ≤ 40%. The questionnaire response rate was 90.9%. A green status was generated for 95.3% of answers. There were 4.5% (n = 1,499) orange alerts and 0.2% (n = 74) red alerts. Overall, 92.1% of patients had at least one CHF related hospitalization and 31.7% (n = 105) of these cases were non-scheduled. The NPV at seven days was 99.43%.
Conclusion: Satelia® Cardio is a feasible, relevant and reliable solution to safely monitor the cohorts of patients with CHF, reassuring cardiologists about patient stability.
BMI: Body Mass Index; BNP: Brain Natriuretic Peptide; CHF: Chronic Heart Failure; CNIL: Commission nationale informatique et liberté (French data protection authority); ESC: European Society of Cardiology; LVEF: Left Ventricular Ejection Fraction; HCP: Healthcare Professional; NPV: Negative Predictive Value; NT-proBNP: N-Terminal pro hormone BNP; OSICAT: Optimisation de la Surveillance Ambulatoire Des Insuffisants CArdiaques Par Télécardiologie (Optimization of the Ambulatory Monitoring for Patients With Heart Failure by Tele-cardiology); PROs: Patient Reported Outcomes; RPM: Remote Patient Monitoring
Remote patient monitoring (RPM) used for chronic diseases is an innovative way to improve patient management and decrease subsequent complications faced by patients. It is a telemedicine activity designed to periodically collect and share relevant medical data and requires the active involvement of both healthcare professionals (HCPs) and patients, often supported by therapeutic coaching.
RPM has shown to improve patient management in various medical specialties including cardiology [1]. For patients with chronic heart failure (CHF), telemedicine use has been supported by the European Society of Cardiology (ESC) since 2019 and was reconfirmed in the latest 2021 update (as a Class IIb recommendation) [2,3]. Its role has been to monitor patient stability and to improve patient management in the case of destabilizing CHF.
To date, RPM for CHF patients has not been entirely scaled in routine practice in Europe mainly due to a lack of adapted funding and reimbursement models [4]. Nevertheless, there have been a few programs aimed at promoting its implementation through dedicated public funding programs such as the national ETAPES program in France which is scheduled to end in 2023 [5,6].
Available real-world evidence has been limited with most studies relying on connected devices employing automated data collection capabilities than on questionnaires concerning symptoms and patient reported outcomes (PROs) [7-10]. The objective of this study was to describe the real-world use of a publicly funded ETAPES program for patients with CHF and to evaluate the performance of Satelia® Cardio clinical algorithm assessing the stability of patients.
A retrospective observational study was conducted on patients with CHF who benefited from an RPM solution (Satelia® Cardio) within the ETAPES funding program in France. Patients were included from September 1, 2018, to June 30, 2020.
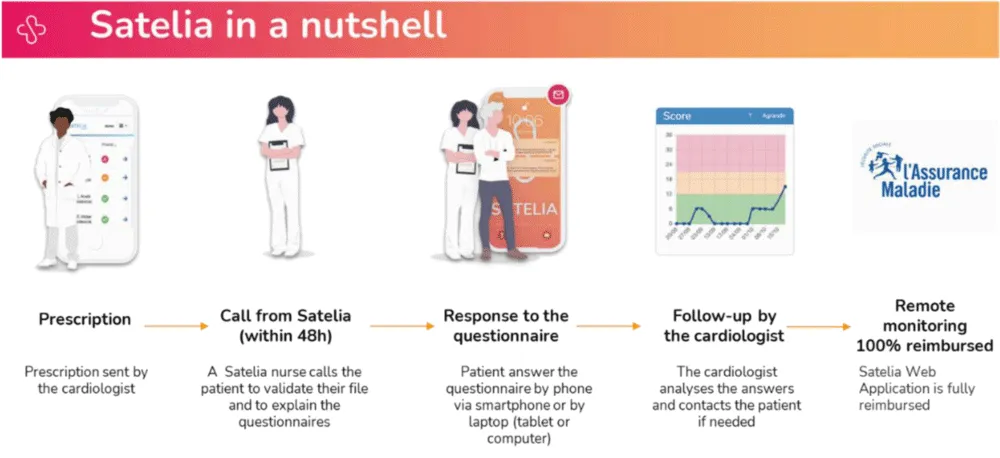
Satelia® Cardio is a telehealth provider which offers a dedicated RPM web application based on PROs, a clinical algorithm based on patient answers and a nursing service for patient follow-ups and education in order to detect situations at risk of decompensation earlier. An overview of the software is shown in Figure 1.
Figure 1: Overview of the remote patient monitoring (RPM) software (Satelia® Cardio web application).
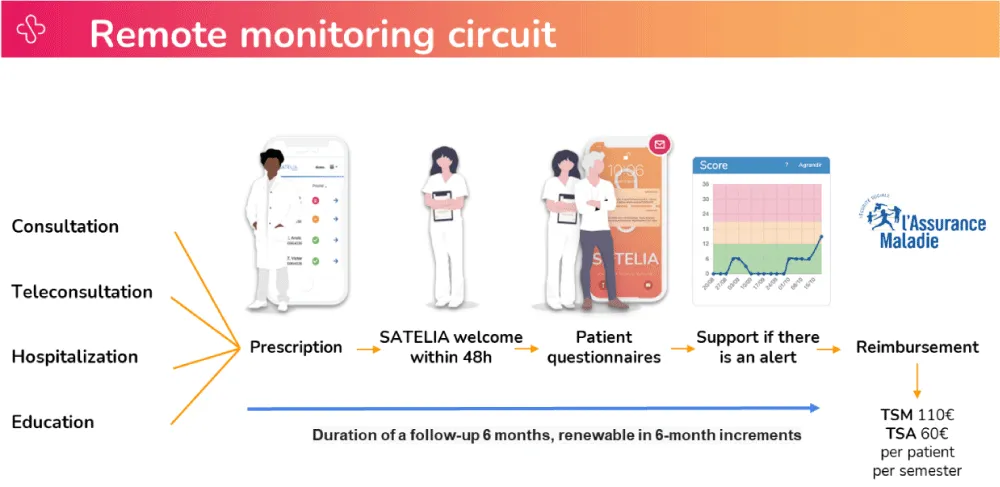
The ETAPES program is a publicly-funded reimbursement plan for RPM in France and builds on a joint capitation funding and payment-for-performance model in which a hospital and a RPM solution provider are reimbursed on a per patient and per semester basis (Figure 2).
Figure 2: Details of the remote monitoring circuit of the Satelia® Cardio web application.
The ETAPES program, inclusion criteria, and the RPM software were described in a previously published article [11,12].
All participating ETAPES program sites and patients treated at these sites were eligible for the study. The number of participating sites was eight. Data were collected from the web application and from the electronic health records kept at the participating sites. Informed consent was needed by all participating patients in addition to consent for the use of RPM (both of which were given at inclusion). Patient follow-up was ended if the inclusion criteria were no longer met (such as patient death, discontinuation of monitoring, or any violations of ETAPES program criteria). At the time of the study and in the ETAPES national program, it was forbidden in France to perform RPM on patients with CHF monitoring through an implantable device. If a patient had a defibrillator, the data collected could not be used for RPM of the CHF patient.
Consecutive patients were included if they had completed at least six months of follow- up. Patients with a follow-up period longer than six months but with no available data were excluded. Patient characteristics at inclusion were age, gender, height, weight and BMI. Medical characteristics at inclusion were left ventricular ejection fraction (LVEF), NYHA, blood pressure, CHF etiology, comorbidities, biological tests such as BNP or NT-proBNP and treatments. Moreover, we wanted to determine the factors favoring alerts by analyzing the impact of the dosage of BNP and/or NT-proBNP at entry of 100 or more than 100 and LVEF ± 40% [13].
Follow-up time in months and follow-up failures were also described. Follow-up started at the inclusion date and ended at data extraction or at any withdrawal date. Compliance was described as the percentage of questionnaires answered during the follow-up period. The treatment regimen of patients was reflective of real-life settings of daily practice as shown in previous literature such as the QUALIFY survey [14] and the VICTORIA study [15]. Due to the nature of this study, patient did not have the up titration yet and neither all the recommended treatments. Depending on the calculated risk from the clinical algorithm, patients were categorized into three levels: green status (no alert), orange alert and red alert. A green status corresponded to a clinical algorithm risk score between 0 and 11, an orange alert corresponded to a score between 12 and 20 and a red alert corresponded to a score between 21 and 36. Data for the orange and red alerts were collected with the physician’s resolution and the delay time from the alert given to the resolution provided by the physician. A patient could have had multiple orange and red alerts during a follow- up.
The algorithm was based on weighted questions and each question had points depending on the answers provided. Answers to the questions related to symptoms were categorized in three levels. Each questions had different weights and each answers had different points depending on the question. The patient weight was included in the algorithm based on the difference between the current weight and reference weight and the algorithm did not rely on simple yes/no answers.
A resolved alert meant that the physician acknowledged and managed it. However, alerts could also be left unresolved. Clinical outcomes were scheduled or nonscheduled CHF related hospitalizations (defined as at least one day of hospitalization in any type of hospital unit, including emergency departments). Scheduled hospitalizations were defined as hospitalizations that were directly in the clinical ward and planned beforehand.
Non-scheduled hospitalizations were defined through the emergency ward, ICU, cardiological UCI or critical care ward and were acute and non-previously programmed for stable patients. In this analysis, if the cause of scheduling information of the hospitalization was not available, the hospitalization was not included.
The performance of the clinical algorithm was evaluated through the negative predictive value (NPV) of the alert generation’s prediction of hospitalization within the seven days after generation and conversely. The positive predictive value (PPV) was not an accurate marker in our study however, can be available upon author request. The study was compliant with all national regulations. An MR-004 declaration was filed with the French data protection authority, Commission nationale informatique et liberté (CNIL), for the reuse of previous data routinely collected by cardiologists.
In total, 360 patients were initially eligible and 331 patients were included in the study. Twenty-nine patients were not included because they did not have a follow-up at 6 or 12 months in the inclusion center. During the course of two consecutive years, 36,682 questionnaires were responded to, with 33,348 of them answered by the patient or nurse and 3,334 questionnaires with no answers. Due to the specific approach adapted to all types of patients with the availability of needed nurse calls, no patients were lost from follow- up. In addition, all hospitalizations were documented with a hospitalization report; therefore, this study did not have hospitalizations excluded due to a lack of scheduling information.
Patient characteristics at inclusion are described in Table 1. The mean age of patients was 68.1 years (digital patients = 64.6 years, patients with poor digital literacy = 75.9 years), were mostly males (70.4%) and had a mean BMI of 27.2 (± 5.4). The mean LVEF was 35.4% (± 12.3) and most patients (73.3%) had LVEF ≤ 40%.
| Table 1: Patient characteristics of a real-world study of remote patient monitoring for chronic heart failure using Satelia® Cardio | |
| Patient Characteristics | Patients N |
| Age | |
| Mean (± SD) Median Min-Max |
68.1 (13.3) 70 20.0-92.0 |
| Sex | |
| Male N (%) | 233 (70.4) |
| Weight (kg) | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
79.2 (18.2) 77.7 (66.0-90.0) 37.0 - 146.0 |
| Height | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
170.4 (8.7) 170.0 (165.0-176.0) 144.0-198.0 |
| BMI | |
| Mean (± SD) | 27.2 (5.4) |
| Median (Q1-Q3) Min-Max | 26.2 (23.4-30.4) 16.4-42.7 |
| LVEF | |
| Mean (± SD) Median (Q1-Q3) Min-Max ≤ 40% 40 ˂ LVEF < 50% ≥ 50% |
35.4 (12.3) 34.0 (25.0-44.0) 10.0-74.0 228 (73.3) 31 (10.0) 52 (16.7) |
| NYHA | |
| II III IV |
154 (65.8) 71 (30.3) 9 (3.8) |
| CHF cause | |
| Ischemic Non-ischemic |
139 (43.3) 182 (56.7) |
| Systolic blood pressure | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
122.6 (21.8) 120.0 (105.0-139.0) 78.0-191.0 |
| Diastolic blood pressure | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
71.9 (13.3) 70.0 (60.0-80.0) 40.0-121.0 |
| Heart rate | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
80.0 (21.6) 74.0 (65.0-91.0) 44.0-160.0 |
| Comorbidities N (%) | |
| Atrial fibrillation High blood pressure Myocardial infarction Stroke Diabetes Liver failure Kidney failure Cancer Pacemaker Defibrillator |
149 (45) 135 (40.8) 74 (22.4) 29 (8.8) 95 (28.7) 1 (0.3) 63 (19) 29 (9) 26 (7.9) 118 (35.9) |
| BNP (pg/mL) | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
802.4 (1,414.4) 420.0 (194.5-936.0) 10.0-16,643.0 |
| NT-proBNP (pg/mL) | |
| Mean (±SD) Median (Q1-Q3) Min-Max |
2,881.1 (3,769.5) 1,535.0 (794.0-3,393.0) 96.0-26,879.0 |
| Creatinine clearance (mL/min/1.73m2) | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
60.0 (25.8) 56.8 (40.0-76.9) 15.0-156.0 |
| Treatment | |
| At least 1 diuretic ACE inhibitors ARA2 Beta-blockers Anti-aldosterone Ivabradine Entresto (sacubitril/valsartan) Digitalis Cordarone Nitrate Derivatives |
272 (82.2) 72 (21.8) 32 (9.7) 289 (87.3) 194 (58.6) 24 (7.3) 175 (52.9) 6 (1.8) 71 (21.5) 1 (0.3) |
In addition, most patients had a NYHA score at II (65.8%). Over half of the patients had non-ischemic CHF (56.7%). The three leading comorbidities were atrial fibrillation (45%), hypertension (40.8%) and diabetes (28.7%). The mean BNP was 802.4 (± 1,414.4) and mean NT-proBNP was 2,881.1 (± 3,769.5). The most commonly prescribed treatments were beta blockers for 87.3% of the total number of patients and 82.2% of patients were treated with at least one diuretic with 58.6% treated with anti-aldosterone. There were 20.8% treated with ACE inhibitors (ACEi), 9.3% treated with angiotensin receptor blockers (ARB) and 60.6 % treated with angiotensin receptor neprilysin inhibitors (ARNi). Among patients with LVEF ≤ 35%, there were 64.6% treated with tritherapy (beta blocker + ACEi-or-ARB-or-ARNi + MRA).
The mean creatinine clearance was 60.0 (± 25.8) and 35.9% of patients had an implantable cardiac defibrillator. In total, the response rate to the questionnaires was 90.9% (95% for digital patients and 77.7% for patients with poor digital literacy, which were 11.3 years older than the digital patients). The mean follow-up duration was 21.4 months (± 6.1) and the minimum duration was 11 months with the maximum at 34.5. In total, 20 patients (6%) prematurely stopped follow-up based on the patient or physician’s decision with respect to the program criteria.
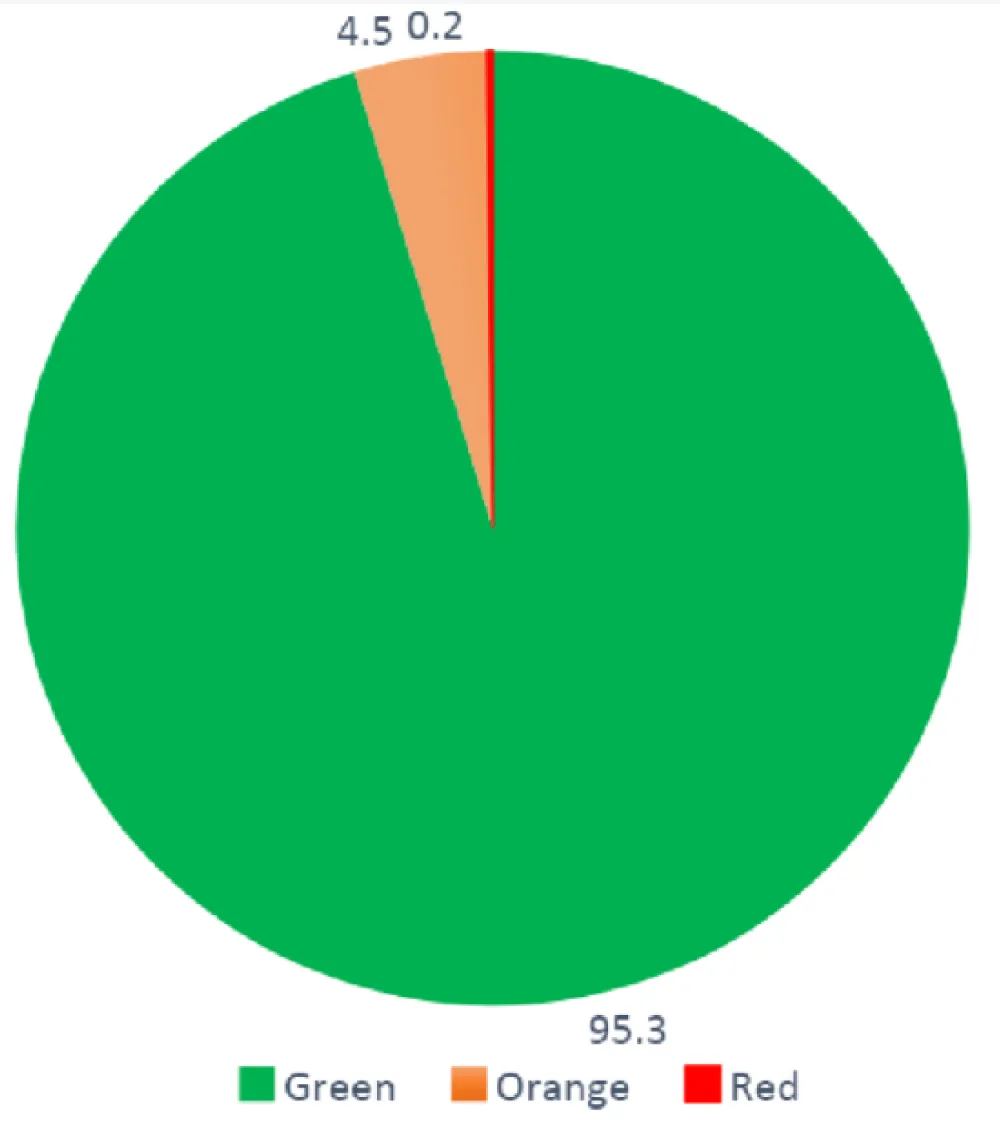
A green status was indicated for 95.3% of patient answers (Table 2) and 4.7% of the patient answers generated alerts of which 4.5% (n = 1,499) were orange alerts and 0.2% (n = 74) red (Figure 3). Among the generated alerts, 47.5% (n = 747) were resolved (47.6% for orange and 45.9% for red alerts). The mean delay by the cardiologist to resolve alerts was 1.1 days (± 1.8) for orange alerts and 0.6 (± 0.9) for red alerts. Among patients with non-resolved alerts, 51.7% of them had a new alert triggered within seven days. The delay for patients with orange alerts going back to a green status was 10 (± 11.4) days if the alert was not resolved and 7.9 (± 9.0) days if the alert was (p = 0.0059).
| Table 1: Patient characteristics of a real-world study of remote patient monitoring for chronic heart failure using Satelia® Cardio | |
| Patient Characteristics | Patients N |
| Age | |
| Mean (± SD) Median Min-Max |
68.1 (13.3) 70 20.0-92.0 |
| Sex | |
| Male N (%) | 233 (70.4) |
| Weight (kg) | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
79.2 (18.2) 77.7 (66.0-90.0) 37.0 - 146.0 |
| Height | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
170.4 (8.7) 170.0 (165.0-176.0) 144.0-198.0 |
| BMI | |
| Mean (± SD) | 27.2 (5.4) |
| Median (Q1-Q3) Min-Max | 26.2 (23.4-30.4) 16.4-42.7 |
| LVEF | |
| Mean (± SD) Median (Q1-Q3) Min-Max ≤ 40% 40 ˂ LVEF < 50% ≥ 50% |
35.4 (12.3) 34.0 (25.0-44.0) 10.0-74.0 228 (73.3) 31 (10.0) 52 (16.7) |
| NYHA | |
| II III IV |
154 (65.8) 71 (30.3) 9 (3.8) |
| CHF cause | |
| Ischemic Non-ischemic |
139 (43.3) 182 (56.7) |
| Systolic blood pressure | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
122.6 (21.8) 120.0 (105.0-139.0) 78.0-191.0 |
| Diastolic blood pressure | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
71.9 (13.3) 70.0 (60.0-80.0) 40.0-121.0 |
| Heart rate | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
80.0 (21.6) 74.0 (65.0-91.0) 44.0-160.0 |
| Comorbidities N (%) | |
| Atrial fibrillation High blood pressure Myocardial infarction Stroke Diabetes Liver failure Kidney failure Cancer Pacemaker Defibrillator |
149 (45) 135 (40.8) 74 (22.4) 29 (8.8) 95 (28.7) 1 (0.3) 63 (19) 29 (9) 26 (7.9) 118 (35.9) |
| BNP (pg/mL) | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
802.4 (1,414.4) 420.0 (194.5-936.0) 10.0-16,643.0 |
| NT-proBNP (pg/mL) | |
| Mean (±SD) Median (Q1-Q3) Min-Max |
2,881.1 (3,769.5) 1,535.0 (794.0-3,393.0) 96.0-26,879.0 |
| Creatinine clearance (mL/min/1.73m2) | |
| Mean (± SD) Median (Q1-Q3) Min-Max |
60.0 (25.8) 56.8 (40.0-76.9) 15.0-156.0 |
| Treatment | |
| At least 1 diuretic ACE inhibitors ARA2 Beta-blockers Anti-aldosterone Ivabradine Entresto (sacubitril/valsartan) Digitalis Cordarone Nitrate Derivatives |
272 (82.2) 72 (21.8) 32 (9.7) 289 (87.3) 194 (58.6) 24 (7.3) 175 (52.9) 6 (1.8) 71 (21.5) 1 (0.3) |
Figure 3: Percentage (%) of total green status, orange and red alerts using the Satelia® Cardio remote monitoring program for patients with chronic heart failure in France.
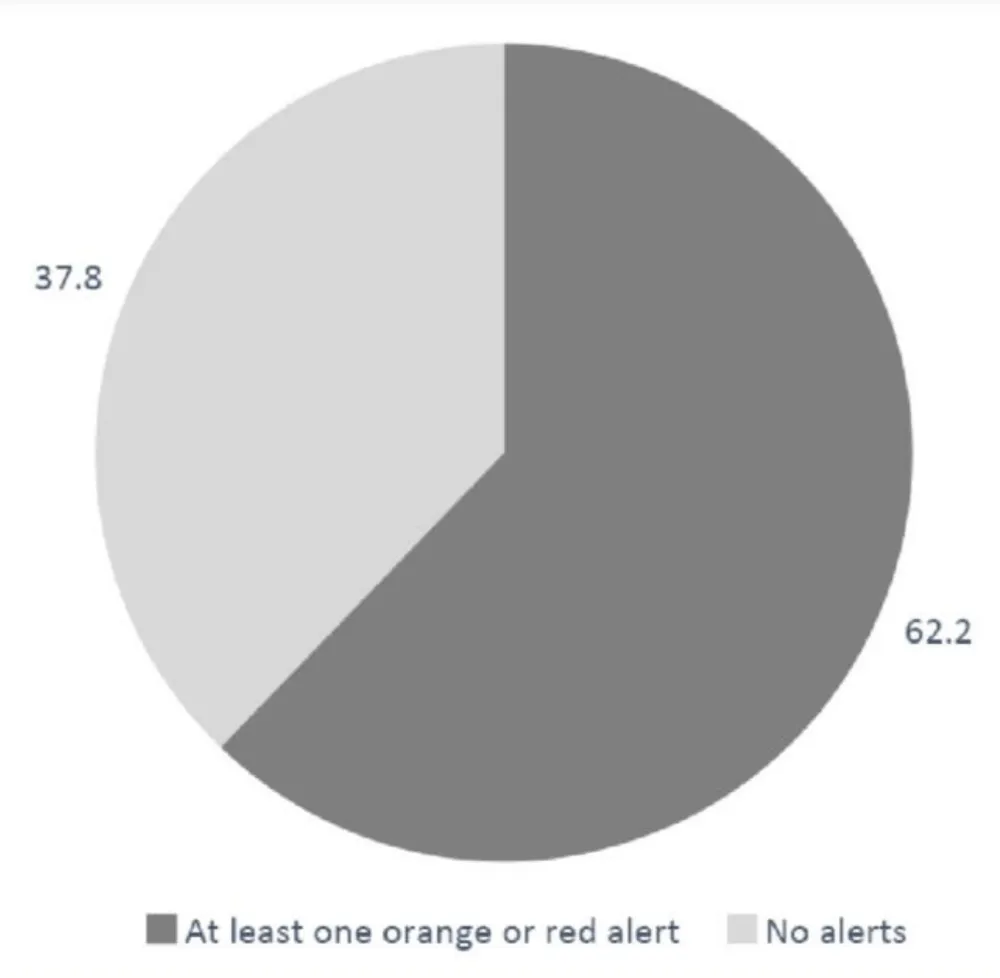
Two-hundred and six patients (62.2%) had at least one alert during their follow-up with a mean of 7.6 (± 9.9) alerts per patient (Figure 4) and 61.9% of patients (n = 205) had at least one orange alert with a mean of 7.3 (± 9.5) alerts per patient. Regarding red alerts, 10.6 % (n = 35) of patients had at least one red alert with a mean of 2.1 alerts per patient (± 1.6). The mean score for the orange alerts was 13.2 (± 2.0) and the mean score for red alerts was 23.1(± 2.7).
Figure 4: Percentage (%) of patients with at least one orange or red alert when using the Satelia® Cardio remote monitoring program for patients with chronic heart failure in France.
Additionally, 92.1% of patients had at least one CHF related hospitalization and 31.7% of these cases (n = 105) were non-scheduled. In the study group, the annual incidence of unscheduled CHF related hospitalization was 37 per 100 patient-year.
Concerning the link between alerts and BNP or LVEF, patients with BNP ≥ 100pg/mL had 34 alerts per month per 100 patients and patients with BNP ≤ 100pg/mL had 23 alerts per month per 100 patients. Patients with LVEF ≤ 40% had a mean of 31 alerts per month, and those with LVEF ≥ 50% had a mean 17 alerts per month (per 100 patients). The NPV at seven days was 99.43%. Whereas, the NPV for scores ≤ 6 was 99.56%, 98.5% for scores between 7 and 9 and 97.16% for scores between 10 and 11. The unscheduled hospitalization rate was 33% in case of a red alert and 3.3% in case of an orange.
This study was the first in France to describe the real-world implementation of an RPM program for patients with CHF based on PROs within the funding model of the ETAPES program. A web application such as Satelia® Cardio proved to be a feasible and relevant tool in routine cardiology practices. The high performance of the clinical algorithm implies that a cardiologist using Satelia® Cardio in their daily clinical practice can be reassured that patients with a green status have a low risk of hospitalization in the week following the transmitted status and should not need any additional support than what is already provided by the application.
This study demonstrated that the absence of alerts was highly predictive of the absence of hospitalizations. Instead of identifying the risk of decompensation, we showed that the system used by the Satelia® Cardio could identify the absence of decompensation when the score is equal or below 11 over 36. This may allow physicians to potentially focus more on patients with orange or red alerts who are at a higher risk of decompensation, intercurrent events or hospitalization, and to better assess their situation and make proper decisions.
Previous studies such as the OSICAT (Optimisation de la Surveillance Ambulatoire Des Insuffisants CArdiaques Par Télécardiologie) compared standard care to RPM care and included daily body weight measurements, daily recording of heart failure symptoms and personalized education, however, it was unable to demonstrate a decrease of unscheduled hospitalizations or all-cause mortality rates [16]. In Germany, the TIM-HF2 study conducted between 2013 and 2017 also found no difference in cardiovascular mortality between two randomized groups despite a decrease in hospitalization rates [17]. Systematic reviews on this topic have also shown heterogeneous results from clinical trials focusing on the prevention of clinical outcomes [7,18]. Limited research has specifically studied the impact of such interventions on the daily routine of physicians [8,19,20].
In this present study, we highlight the need to carefully design future studies on RPM based on clinical and public health objectives in the context of a digital health intervention [21]. The largest real-world evaluation of RPM for patients with CHF was recently published with this approach and it retrospectively included 659 patients between 2009 and 2016 before the start of the ETAPES funding program and showed a decrease in the hospitalization rate at 12 months with an adherence dose effect. Patients with a higher adherence to RPM had better outcomes than those with lower adherence [22].
Our questionnaire response rate was high for connected patients which may show the ease in which patients older than 65 years can complete online questionnaires on a regular basis. Moreover, this shows that larger scale deployment of an RPM solution can be performed, especially following the introduction of routine RPM funding in France from 2023 [23].
Our findings were in the higher range of observance rates compared to that reported in a recent systematic review of between 77% and 99% [24]. A low compliance rate may reflect the practical difficulty faced by nurses to reach patients by phone to answer questions on time since patients were older in age, less equipped with a personal mobile phone and with a higher probability to be hospitalized/outside their home for healthcare needs.
However, even though this situation could be the consequence of a patient’s age or chronic disease burden, the system used in our study allowed to include all patients from the RPM activity even if a patient had poor digital literacy. This may show that tools such as Satelia® Cardio can support better care equity. Additionally, the rate of patients who withdrew from the follow-up was relatively low, which can be associated with the very high compliance rate to accurately assess the potential impact of the RPM solution.
The proportion of patients who triggered an alert was relatively low even though they represented a population not particularly at lower risk of CHF than the general CHF patient population. Even if patients had NYHA 2 status, they also had comorbidities and low LVEF.
The rate of patients with at least one alert during their follow-up (62.2%) with a mean of 7.6 (± 9.9) alerts per patient was relatively similar to the rate from the SCAD study of 67.5% with a mean of 5.1 (± 5.0) alerts per patient [20]. Among these alerts, most were not resolved by the physicians. This could question the ability of physicians to safely detect events that present higher risks of complications; however, it also shows the reality of the real-life behaviors of HCPs. Additionally, it is worth noting that even patients with high LVEF or low BNP had alerts, meaning that the patients considered as low risk of acute events could also benefit from this follow-up. Among the unresolved alerts, it could be hypothesized that some alerts were not seen on time by the physicians due to their workload, or they may have waited to see if other alerts would be triggered over time before actually resolving them based on their clinical expertise.
The main strengths of this study were the high number of participants, the real-world setting, and the multicenter design covering various regions in France. It may be worth noting that patient follow-up procedures conducted during the COVID-19 pandemic lockdowns did not cause specific problems to the program and may reflect a strength to the study.
The main limitation resided in not having a control group. However, due to the nature of the national ETAPES program and the challenges of its practical and ethical implementation, such a study would have been highly difficult to conduct. Adding a control group and/or a before-after comparison between the groups of patients could be considered in the future however, it was not relevant nor feasible for this present study since we wanted to first descriptively assess the algorithm performance to predict patient stability. We also wanted to validate the performance of an algorithm in real life so that we could identify low-risk HF patients before performing a clinical study. Research on the before-after comparison is planned to be performed in the future, however, it may need consideration since treatment of patients evolve after hospitalization and having this comparison will incur biases.
A cost-utility analysis of RPM versus standard care covering healthcare payers direct, indirect and informal care costs were not performed, however, a cost-effectiveness and cost- utility study is ongoing. Moreover, even though a randomized clinical trial is relevant, we wanted to first validate the real-life performance of an algorithm to identify patients at risk (high and low). We applied a similar methodology used in Boehmer, et al. [25] to avoid the loss of chance for patients, however a randomized trial in the future to formally test the app should be performed as shown in other medical specialties in Giallauria, et al. [26].
It may also be worth noting that we only reported the hospitalization rates and did not provide how often the hospitalization was decided by the referring cardiologist which may have posed a limitation.
The results of a recent large scale RPM study (related to COVID-19) support the need to reevaluate the impact of using RPM on daily clinical practice in addition to how to transition from the national ETAPES program to a more sustainable approach in France [27-29]. These non-invasive programs relying only on PROs coupled with an algorithm and therapeutic patient education may allow for safe early hospital discharge and potentially reduce hospital-related complications, especially in elderly patients, without experiencing any logistics difficulties in the deployment of connected devices in patients’ homes [30,31]. This model may even reach to remotely managed ambulatory IV diuretic management after early patient discharge [32].
Scaling-up RPM programs in a short period of time could be easily conducted without heavy logistics and maintenance. However, for acute decompensation events for patients with CHF, being able to monitor oxygen or blood pressure by connected devices may be more relevant and feasible on targeted patients rather than on a whole cohort. These measurements could be performed by nurses coming to the patient’s home on a needs basis therefore limiting the deployment of devices at home and making the measurement more reliable.
To our knowledge, this study was a paradigm shift in RPM research and evaluation. We showed that a key objective of RPM may be to support and reassure physicians by identifying low-risk patients. This may help physicians to better manage cohorts remotely by relying on a system that ensures continuous patient follow-up and education at scale. In daily practice for cardiologists, this could mean better tailored and personalized consultations with the right patient at the right time without discrimination. A comparison of various models of RPM will be necessary to perform in the future that supports the definition of and routine expansion of any adapted funding model related to patient care pathways.
Declarations
Ethics approval: An MR-004 declaration to CNIL was done for secondary use of previously collected data. No other ethics declaration was required according to French national regulations.
Competing interests: The authors declare no competing interests in relationship with this manuscript: P Jourdain, F Picard, N Girerd, S Lafitte, H Lemieux, F Barritault, MF Seronde, J Ph. Labarre and B Diebold. N. Pages is founder and CEO of NP Medical. H Chaouky, L Betito and C Bedel are employees of NP Medical S Nisse-Durgeat is an employee of WeHealth™ Digital Medicine/Servier.
Funding: This study was funded by WeHealth™ Digital Medicine.
Author’s contributions: P Jourdain, N Pages and S Nisse-Durgeat contributed to the writing of the manuscript. P Jourdain, N Pages, S Nisse-Durgeat, H Chaouky, C Bedel and L Betito contributed to the writing of the study protocol. C Bedel and L Betito: reviewed the manuscript. P Jourdain, F Picard, N Girerd, S Lafitte, H Lemieux, F Barritault, MF Seronde, JPh. Labarre, H Chaouky, B Diebold: usage and inclusion of patients in Satelia® Cardio.
The author would like to thank AcaciaTools for their medical writing and reviewing services.
- Kinast B, Lutz M, Schreiweis B. Telemonitoring of Real-World Health Data in Cardiology: A Systematic Review. Int J Environ Res Public Health. 2021 Aug 27;18(17):9070. doi: 10.3390/ijerph18179070. PMID: 34501659; PMCID: PMC8431660.
- Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019 Oct;21(10):1169-1186. doi: 10.1002/ejhf.1531. Epub 2019 Aug 30. PMID: 31129923.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-3726. doi: 10.1093/eurheartj/ehab368. Erratum in: Eur Heart J. 2021 Oct 14;: PMID: 34447992.
- Tromp J, Jindal D, Redfern J, Bhatt A, Séverin T. World Heart Federation Roadmap for Digital Health in Cardiology. Global Heart. 2022; 17(1): 59.
- Le Douarin Y, Traversino Y, Graciet A, Josseran A; participants of Giens XXXV Round Table Health, economy. Telemonitoring and experimentation in telemedicine for the improvement of healthcare pathways (ETAPES program). Sustainability beyond 2021: What type of organisational model and funding should be used? Therapie. 2020 Jan-Feb;75(1):43-56. doi: 10.1016/j.therap.2019.12.009. Epub 2019 Dec 20. PMID: 32014299.
- Pages N, Picard F, Barritault F, Amara W, Lafitte S, Maribas P, Abassade P, Labarre JP, Boulestreau R, Chaouky H, Abdennadher M, Lemieux H, Lasserre R, Bedel C, Betito L, Nisse-Durgeat S, Diebold B. Remote patient monitoring for chronic heart failure in France: When an innovative funding program (ETAPES) meets an innovative solution (Satelia® Cardio). Digit Health. 2022 Aug 22;8:20552076221116774. doi: 10.1177/20552076221116774. PMID: 36034602; PMCID: PMC9403459.
- Auener SL, Remers TEP, van Dulmen SA, Westert GP, Kool RB, Jeurissen PPT. The Effect of Noninvasive Telemonitoring for Chronic Heart Failure on Health Care Utilization: Systematic Review. J Med Internet Res. 2021 Sep 29;23(9):e26744. doi: 10.2196/26744. PMID: 34586072; PMCID: PMC8515232.
- Faragli A, Abawi D, Quinn C, Cvetkovic M, Schlabs T, Tahirovic E, Düngen HD, Pieske B, Kelle S, Edelmann F, Alogna A. The role of non-invasive devices for the telemonitoring of heart failure patients. Heart Fail Rev. 2021 Sep;26(5):1063-1080. doi: 10.1007/s10741-020-09963-7. PMID: 32338334; PMCID: PMC8310471.
- Boiteux MC, Rey P, Cadiou F, Chauvet C. Un service de télésurveillance et de coordination des soins de l’insuffisant cardiaque [Cardiauvergne is a remote monitoring and care coordination service for patients with severe heart failure]. Soins. 2016 Nov;61(810):45-47. French. doi: 10.1016/j.soin.2016.09.009. PMID: 27894480.
- Andrès E, Talha S, Zulfiqar AA, Hajjam M, Ervé S, Hajjam J, Gény B, Hajjam El Hassani A. Current Research and New Perspectives of Telemedicine in Chronic Heart Failure: Narrative Review and Points of Interest for the Clinician. J Clin Med. 2018 Dec 13;7(12):544. doi: 10.3390/jcm7120544. PMID: 30551588; PMCID: PMC6306809.
- Picard F. Barritault F, Amara W, Lafitte S, Maribas P. First experience with a ready to use solution for remote monitoring of patients suffering from heart failure in France. Archives of Cardiovascular Diseases Supplements. 2021; 13(1):26.
- Desnos M, Jourdain P. Telemedicine: a promising solution for heart failure?. Bulletin de l’Académie Nationale de Médecine. 2020; 204: 817-825.
- Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, Aupetit JF, Aumont MC, Galinier M, Eicher JC, Cohen-Solal A, Juillière Y. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007 Apr 24;49(16):1733-9. doi: 10.1016/j.jacc.2006.10.081. Epub 2007 Apr 2. PMID: 17448376.
- Komajda M, Anker SD, Cowie MR, Filippatos GS, Mengelle B, Ponikowski P, Tavazzi L; QUALIFY Investigators. Physicians' adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail. 2016 May;18(5):514-22. doi: 10.1002/ejhf.510. Epub 2016 Apr 20. PMID: 27095461.
- Senni M, Alemayehu WG, Sim D, Edelmann F, Butler J, Ezekowitz J, Hernandez AF, Lam CSP, O'Connor CM, Pieske B, Ponikowski P, Roessig L, Voors AA, Westerhout CM, McMullan C, Armstrong PW; VICTORIA Study Group. Efficacy and safety of vericiguat in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan: insights from the VICTORIA trial. Eur J Heart Fail. 2022 Sep;24(9):1614-1622. doi: 10.1002/ejhf.2608. Epub 2022 Jul 20. PMID: 35791083.
- Galinier M, Roubille F, Berdague P, Brierre G, Cantie P, Dary P, Ferradou JM, Fondard O, Labarre JP, Mansourati J, Picard F, Ricci JE, Salvat M, Tartière L, Ruidavets JB, Bongard V, Delval C, Lancman G, Pasche H, Ramirez-Gil JF, Pathak A; OSICAT Investigators. Telemonitoring versus standard care in heart failure: a randomised multicentre trial. Eur J Heart Fail. 2020 Jun;22(6):985-994. doi: 10.1002/ejhf.1906. Epub 2020 Jun 15. PMID: 32438483.
- Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018 Sep 22;392(10152):1047-1057. doi: 10.1016/S0140-6736(18)31880-4. Epub 2018 Aug 25. PMID: 30153985.
- Bashi N, Karunanithi M, Fatehi F, Ding H, Walters D. Remote Monitoring of Patients With Heart Failure: An Overview of Systematic Reviews. J Med Internet Res. 2017 Jan 20;19(1):e18. doi: 10.2196/jmir.6571. PMID: 28108430; PMCID: PMC5291866.
- Mhanna M, Beran A, Nazir S, Al-Abdouh A, Barbarawi M, Sajdeya O, Srour O, Altujjar M, Patel RB, Eltahawy EA. Efficacy of remote physiological monitoring-guided care for chronic heart failure: an updated meta-analysis. Heart Fail Rev. 2022 Sep;27(5):1627-1637. doi: 10.1007/s10741-021-10176-9. Epub 2021 Oct 5. PMID: 34609716.
- Cuba Gyllensten I, Crundall-Goode A, Aarts RM, Goode KM. Simulated case management of home telemonitoring to assess the impact of different alert algorithms on work-load and clinical decisions. BMC Med Inform Decis Mak. 2017 Jan 17;17(1):11. doi: 10.1186/s12911-016-0398-9. PMID: 28095849; PMCID: PMC5240411.
- Umeh CA, Reddy M, Dubey A, Yousuf M, Chaudhuri S, Shah S. Home telemonitoring in heart failure patients and the effect of study design on outcome: A literature review. J Telemed Telecare. 2021 Aug 9:1357633X211037197. doi: 10.1177/1357633X211037197. Epub ahead of print. PMID: 34369171.
- Sabatier R, Legallois D, Jodar M, Courouve L, Donio V, Boudevin F, De Chalus T, Hauchard K, Belin A, Milliez P. Impact of patient engagement in a French telemonitoring programme for heart failure on hospitalization and mortality. ESC Heart Fail. 2022 Oct;9(5):2886-2898. doi: 10.1002/ehf2.13978. Epub 2022 Jun 17. PMID: 35715956; PMCID: PMC9715861.
- High Authority of Health. Medical remote monitoring of chronic heart failure patients, Repository of care functions and organizations for medical remote monitoring solutions for chronic heart failure patients. Paris. https://www.has-sante.fr/upload/docs/application/pdf/2022-01/avis_referentiel_insuffis%20ance_cardiaque_chronique_2022-01-24_09-34-42_519.pdf
- Morken IM, Storm M, Søreide JA, Urstad KH, Karlsen B, Husebø AML. Posthospitalization Follow-Up of Patients With Heart Failure Using eHealth Solutions: Restricted Systematic Review. J Med Internet Res. 2022 Feb 15;24(2):e32946. doi: 10.2196/32946. PMID: 35166680; PMCID: PMC8889479.
- Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, An Q, Averina V, Stolen CM, Thakur PH, Thompson JA, Wariar R, Zhang Y, Singh JP. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results From the MultiSENSE Study. JACC Heart Fail. 2017 Mar;5(3):216-225. doi: 10.1016/j.jchf.2016.12.011. PMID: 28254128.
- Giallauria F, Baratta R, Costa F, D'Amario D, DE Gennaro L, Giubilato S, Mattina A, Provenzano M, Santoro D, Versaci F. New paradigm for the management of cardio-nephro-metabolic syndrome: multidisciplinary approach and role of telemedicine. Minerva Med. 2022 Oct 18. doi: 10.23736/S0026-4806.22.08165-4. Epub ahead of print. PMID: 36255711.
- Jourdain P, Artigou JY, Hryschyschyn N, Berthelot E, Bailly MT, Dinh A, Assayag P. La télémédecine, d'ETAPES à COVIDOM …vers une nouvelle ère ? [Telemedicine from experimentation (ETAPES) to COVIDOM… a new era ?]. Ann Cardiol Angeiol (Paris). 2021 Nov;70(5):317-321. French. doi: 10.1016/j.ancard.2021.09.016. Epub 2021 Oct 7. PMID: 34627623.
- Yordanov Y, Dinh A, Bleibtreu A, Mensch A, Lescure FX, Debuc E, Jourdain P, Jaulmes L, Dechartres A; AP-HP/Universities/Inserm COVID-19 research collaboration. Clinical characteristics and factors associated with hospital admission or death in 43 103 adult outpatients with coronavirus disease 2019 managed with the Covidom telesurveillance solution: a prospective cohort study. Clin Microbiol Infect. 2021 Aug;27(8):1158-1166. doi: 10.1016/j.cmi.2021.04.010. Epub 2021 Apr 27. PMID: 33915287; PMCID: PMC8076762.
- Yordanov Y, Dechartres A, Lescure X, Apra C, Villie P, Marchand-Arvier J, Debuc E, Dinh A, Jourdain P; AP-HP / Universities / Inserm COVID-19 Research Collaboration. Covidom, a Telesurveillance Solution for Home Monitoring Patients With COVID-19. J Med Internet Res. 2020 Oct 22;22(10):e20748. doi: 10.2196/20748. PMID: 33006938; PMCID: PMC7644373.
- Dinh A, Mercier JC, Jaulmes L, Artigou JY, Juillière Y, Yordanov Y, Jourdain P; AP-HP/Universities/INSERM COVID-19 Research Collaboration. Safe Discharge Home With Telemedicine of Patients Requiring Nasal Oxygen Therapy After COVID-19. Front Med (Lausanne). 2021 Nov 3;8:703017. doi: 10.3389/fmed.2021.703017. PMID: 34805196; PMCID: PMC8595095.
- Veenis JF, Radhoe SP, Hooijmans P, Brugts JJ. Remote Monitoring in Chronic Heart Failure Patients: Is Non-Invasive Remote Monitoring the Way to Go? Sensors (Basel). 2021 Jan 28;21(3):887. doi: 10.3390/s21030887. PMID: 33525556; PMCID: PMC7865348.
- Girerd N, Mewton N, Tartière JM, Guijarro D, Jourdain P, Damy T, Lamblin N, Bayes-Génis A, Pellicori P, Januzzi JL, Rossignol P, Roubille F; a panel of multidisciplinary experts and the Heart Failure Working Group of the French Society of Cardiology. Practical outpatient management of worsening chronic heart failure. Eur J Heart Fail. 2022 May;24(5):750-761. doi: 10.1002/ejhf.2503. Epub 2022 Apr 27. PMID: 35417093; PMCID: PMC9325366.