More Information
Submitted: May 26, 2023 | Approved: June 19, 2023 | Published: June 20, 2023
How to cite this article: Gaur KK, Ghotekar LH, Margekar SL, Kumar T, Singh R. Vitamin D Deficiency and its Correlation with the Severity of Heart Disease in Dilated Cardiomyopathy Patients. J Cardiol Cardiovasc Med. 2023; 8: 059-064.
DOI: 10.29328/journal.jccm.1001154
Copyright License: © 2023 Gaur KK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Dilated cardiomyopathy (DCMP); New York Heart Association (NYHA); Ejection fraction (E.F.), % fractional shortening (%F.S.)
Vitamin D Deficiency and its Correlation with the Severity of Heart Disease in Dilated Cardiomyopathy Patients
Kuldeep Kumar Gaur1*, LH Ghotekar2, Shubha Laxmi Margekar3, Tarun Kumar4 and Ritu Singh5
1Post Graduate, Department of Medicine, Lady Hardinge Medical College and Associated Hospitals, India
2Director, Professor, Department of Medicine, Lady Hardinge Medical College and Associated Hospitals, India
3Professor, Department of Medicine, Lady Hardinge Medical College and Associated Hospitals, India
4Professor, Department of Cardiology, Atal Bihari Vajpayee Institute of Medical Sciences and Dr. RML Hospital, India
5Director, Professor, Department of Biochemistry, Lady Hardinge Medical College and Associated Hospitals, India
*Address for Correspondence: Kuldeep Kumar Gaur, Post Graduate, Department of Medicine, Lady Hardinge Medical College and Associated Hospitals, India, Email: [email protected]
Background: Cardiomyopathy is primarily a disorder of the cardiac muscle that causes myocardial dysfunction and is not the result of disease or dysfunction of other cardiac structures, systemic arterial hypertension and valvular stenosis or regurgitation.
Aim: The present study aimed to determine the prevalence of vitamin D deficiency and its correlation with the severity of heart disease in patients with dilated cardiomyopathy (DCMP).
Method: 70 ECHO-proven DCMP cases were enrolled from the medicine/ cardiology department of LHMC & associated hospitals and ABVIMS & Dr. RML Hospital, New Delhi from November 2019 to October 2021. DCMP patients with ages more than 18 years who were willing to give consent and does not meet any of the exclusion criteria were enrolled in this study.
Results: Mean age of idiopathic DCMP patients was 48.3 ± 15.2. There were more males 48 (69%) than females 22 (31%). The mean ejection fraction was 26.6 ± 7.3, while the mean fractional shortening was 17.6 ± 3.1. Vitamin D deficiency was observed in 90% of patients, among which 68.5% were having moderate vitamin D deficiency and 10% were having severe vitamin D deficiency.
Conclusion: In our study, vitamin D levels were inversely correlated with the severity of heart disease in DCMP patients.
DCMP is a progressive disease of the heart muscle i.e. a group of myocardial disorders characterized by dilatation of ventricles, contractile dysfunction of the left ventricle in the absence of chronic pressure and/or volume overload, and systolic dysfunction [1]. The 1980 WHO committee reserved the term cardiomyopathy for myocardial disease of unknown cause, but now there are multiple defined mechanisms e.g. ischemic, toxic, immunological, and metabolic, damaging the heart muscle leading to a common final outcome, represented as dilated cardiomyopathy. It occurs more commonly in males. It predominates in middle age and elderly age groups [2].
With the rapid advancement in molecular genetics and uncovering of underlying etiologies, DCMP is being recognized as a specific diagnosis and not one of exclusion. Due to increased awareness of this condition along with improvement in diagnostic techniques, it now accounts for the majority of all causes of heart failure. Depending on the diagnostic criteria used, the reported annual incidence varies between 5-8 cases per 1,00,000 population [3] and a prevalence of 1 case per 2700 (36 cases per 1,00,000) general population, accounting for 10,000 deaths and 46,000 hospitalizations in the United States annually [4].
Recently, the MOGE(S) classification system based on phenotype and genotype has been proposed which incorporates information on structural and functional abnormalities (M), organ involvement (O), genetics (G), etiology (E), and disease severity (S). However, it does not include certain cardiomyopathies as post-partum cardiomyopathy (PPCM) or risk of sudden death and is complex to use [5]. Idiopathic dilated cardiomyopathy (IDC) is essentially an exclusion diagnosis in patients with heart failure, who have met echo diagnostic criteria of DCMP without any obvious cause, as mentioned in the MOGE(S) classification. The clinical profile of DCMP at the time of presentation is highly variable, extending from no/minimal symptoms to progressive refractory heart failure.
Besides above mentioned aetiologies/ mechanisms, the role of nutritional deficiencies is also documented. Hypocalcemic cardiomyopathy occurs possibly via a direct effect on cardiac myocyte contractility. It usually responds favorably to the restoration of normocalcemia but is refractory to the conventional management approach of heart failure [6]. Vitamin D also contributes to heart failure and cardiomyopathy by causing hypocalcemia [7]. In view of the high prevalence of vitamin D deficiency and chronic heart failure due to underlying DCMP with the lack of data on DCMP and possible prevention & treatment of these nutritional factors, this study is being done to determine the role of vitamin D deficiency in DCMP patients.
Our study was a hospital-based cross-sectional study conducted in Lady Hardinge Medical College & associated hospitals and ABVIMS & Dr. Ram Manohar Lohia Hospital, New Delhi. 70 ECHO-proven DCMP cases of age more than 18 years were taken from the medicine/ cardiology ward/ opd of LHMC & associated hospitals and ABVIMS & Dr. RML Hospital, New Delhi from November 2019 to October 2021. Patients who had other serious co-morbid medical or surgical illnesses (sepsis, septic shock, Bariatric surgery, etc.), malabsorption syndrome, ulcerative colitis, and renal failure or who were already diagnosed with cancer or on cardiotoxic drugs such as doxorubicin, 5-FU, methotrexate, were excluded.
Each patient was then subjected to comprehensive clinical history taken by recall method along with the history of associated risk factors to rule out all possible etiologies to classify for idiopathic DCMP. The patients were then subjected to detail clinical examination followed by Chest X-Ray PA view, ECG, and Echo. Routine blood examinations including complete hemogram, liver function test, kidney function test, lipid profile, serum vitamin D, vitamin B12, calcium, and magnesium levels. Vitamin D detection was done with the chemilumiscence method on the Beckman Coulter DXI analyzer using commercial kits from Beckman Coulter.
ECHO
Transthoracic echocardiography (TTE) and Doppler studies were performed using machine Philips HD 11 XE. All standard (parasternal long axis and short axis, apical and subxiphoid) views were done. Also, doppler studies were performed and blood flow was recorded across all four valves. The following parameters were recorded: left ventricular dimension (LVEDD, LVESD), atrial dimension, left ventricular ejection fraction, left ventricular diastolic dysfunction (LVDD), and valvular regurgitation. LV systolic function was assessed by ejection fraction measured in M-mode at the mid-cavity level calculated by the software program built-in the echocardiography machine (normal range 50% - 70%).
Statistical analysis
The data was entered in Excel Spreadsheet and analysis was done using Stata (Texas, version 16.0). Continuous variables were represented as mean ± SD or median with IQR (Inter-quartile range). Categorical variables were represented as numbers and percentages (%).
The variables were tested for normality by the Kolmogorov-Smirnof normality test. All tests of significance were 2-tailed. The Chi2 test was used to check the association of categorical variables. Student t-test was used for checking the association between 2 continuous variables in a normal distribution. For non-parametric distribution, the wilcoxon rank sum test was used for testing this type of association. A sign rank test was used to check the association between 2 paired continuous variables. The strength of association was assessed using the Spearman correlation test. A p - value of < 0.05 was considered statistically significant.
Ethical statement
This study was approved by the institutional ethics committee, LHMC, New Delhi (No. 2019/39). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved Informed consent was taken from all the patients.
Among 70 DCMP patients, 48 (69%) were males and 22 (31%) were females. The mean age of DCMP patients was 48.3 ± 15.2 with 21 (30%) patients lie in the 41-50 years age group, followed by 17 (24.3%), 12 (17.1%), 9 (12.9%), and 11 (15.7%) in > 60, 51-60, 31-40 and < 30 year age group respectively. Mean BMI was 2s3.64 ± 2.57 kg/m2. 40%, 34.3%, and 25.7% of DCMP patients were normal, overweight, and obese respectively.
All patients in this study were symptomatic i.e. having breathing difficulty (100%). Other symptoms in decreasing order of frequency were swelling of feet (85.7%), fatigue (50%), orthopnea (47.1%), paroxysmal nocturnal dyspnea (35.7%), palpitation (35.7%), chest pain (20%), and syncope (5.7%). Maximum patients of both genders had NYHA class IV dyspnea at presentation followed by NYHA III with a p - value of 0.06 (Table 1). Mean respiratory rate, pulse rate, and systolic and diastolic blood pressure were 24.65 ± 5.01 /min., 100.92 ± 14.66 /min., 111.85 ± 17 mm of Hg and 70.71 ± 12 mm of Hg respectively (Table 2). Hypertension was observed in 10%, diabetes in 14.3%, a history of alcohol (non-cirrhotic) in 4.3%, and a family history of DCMP in 2.9% of patients.
| Table 1: Clinical profile of DCMP patients (n = 70). | |||||
| Male (n = 48) | Female (n = 22) | Total n = 70) | P - value | ||
| Breathlessness | No | 0 | 0 | 0 | 0.6 |
| Yes | 48 (69%) | 22 (31%) | 70 (100%) | ||
| PND | No | 31 (68.9%) | 14 (31.1%) | 45 (64.3%) | 0.94 |
| Yes | 17 (68%) | 8 (32%) | 25 (35.7%) | ||
| Orthopnoea | No | 26 (70.3%) | 11 (29.7%) | 37 (52.9%) | 0.75 |
| Yes | 22 (66.7%) | 11 (33.3%) | 33 (47.1%) | ||
| Palpitation | No | 33 (73.3%) | 12 (26.7%) | 45 (64.3%) | 0.25 |
| Yes | 15 (60%) | 10 (40%) | 25 (35.7%) | ||
| Chest pain | No | 39 (69.6%) | 17 (30.4%) | 56 (80%) | 0.70 |
| Yes | 9 (64.3%) | 5 (35.7%) | 14 (20%) | ||
| Swelling feet / Pedal oedema | No | 6 (60%) | 4 (40%) | 10 (14.3%) | 0.53 |
| Yes | 42 (70%) | 18 (30%) | 60 (85.7%) | ||
| Syncope | No | 45 (68.8%) | 21 (31.8%) | 66 (94.3%) | 0.78 |
| Yes | 3 (75%) | 1 (25%) | 4 (5.7%) | ||
| Fatigue | No | 22 (62.9%) | 13 (37.1%) | 35 (50%) | 0.30 |
| Yes | 26 (74.3% | 9 (25.7%) | 35 (50%) | ||
| Table 2: General examination parameters of DCMP patients (n = 70). | |||||||
| Para Meters | Male (n = 48) | Female (n = 22) | Total (n = 70) | ||||
| Mean | SD | Mean | SD | Mean | SD | Median (IQR) | |
| RR (/min) | 25.64 | 5.93 | 22.5 | 8.28 | 24.65 | 5.01 | 26 (20-28.75) |
| PR (/min) | 103.04 | 13.22 | 96.31 | 16.77 | 100.92 | 14.66 | 98.5 (90.25-112) |
| SBP (mmHg) | 111.70 | 15.7 | 112.18 | 19.9 | 111.85 | 17 | 110 (100-120) |
| DBP (mmHg) | 70.79 | 13.2 | 70.54 | 9.1 | 70.71 | 12 | 70 (66-80) |
CP angle blunting was observed in 61 (87.1%) patients with a p - value of 0.52. There was evidence of cardiomegaly in 68 (97.1%) patients with a mean cardiothoracic ratio of 0.58 ± 0.02 with a p - value of 0.23. Sinus rhythm, normal axis, arrhythmia, bundle branch block, and ventricular hypertrophy were observed in 48 patients (68.6%), 41 patients (58.6%), 14 patients (20%), 18 patients (25.7%) and 11 patients (15.7%) respectively. All the patients included in this study met the echo diagnostic criteria of DCMP. The mean ejection fraction was 26.6 ± 7.3 with a p - value of 0.23, while the mean fractional shortening was 17.6 ± 3.1 with a p - value of 0.59. Among these 70 DCMP patients, 42 (60%) patients had E.F. of < 30%, while 18 (25.7%) patients had their E.F. b/w 30% - 39% category and 10 (14.3%) patients had E.F. b/w 40% - 45% with a p - value of 0.34. And 14 (20%) patients had < 15% F.S., 35 (50%) patients had F.S. b/w 15-19.9%, and 21 (30%) patients had F.S. b/w 20-24% with a p - value of 0.67 (Table 3).
| Table 3: Echo findings in DCMP patients. | ||||
| Male (n = 48) | Female (n = 22) | Total (n = 70) | p - value | |
| Ejection fraction | 25.9 ± 6.86 | 28.1 ± 8.14 | 26.6 ± 7.3 | 0.23 |
| Fractional shortening | 17.5 ± 2.9 | 17.9 ± 3.5 | 17.6 ± 3.1 | 0.59 |
| ESD | 4.49 ± 0.73 | 4.84 ± 0.63 | 4.60 ± 0.71 | 0.52 |
| EDD | 5.4 ± 0.87 | 5.9 ± 0.73 | 5.59 ± 0.85 | 0.04 |
The mean Hb was 11.6 ± 2.6 g/dl with anemia observed in 72.9% of patients. The mean TLC was 9578.0 ± 3995.1/mm3 and the mean platelet was 2.2 ± 0.9 lakh/mm3. Mean bilirubin levels were 1.1 ± 0.8 mg/dl. Mean serum albumin was 3.5 ± 0.5g/dl with hypoalbuminemia observed in 30% of patients with a p - value of 0.18. Leucocytosis, deranged LFT, and deranged KFT were observed in 25.7%, 20%, and 37.1% of DCMP patients respectively in our study. Median (IQR) serum AST and ALT values were 36 (27.3-53.8) IU/L and 37 (28-52) IU/L respectively. Median (IQR) serum urea and creatinine levels were 42 (36-55.25) mg/dl and 1 (0.8-1.27) mg/dl respectively. Mean TSH values were 4.5 ± 2.7 IU/ml with hypothyroidism observed in 5.7% of patients. Mean HDL levels were 36.2 ± 11.6 mg/dl, while mean LDL, TG, and cholesterol levels were 99.7 ± 49.7 mg/dl, 126.1 ± 71.8 mg/dl, and 158.1 ± 55 mg/dl respectively. Also, median (IQR) levels of HDL, LDL, T.G., and cholesterol were 37.5 (31-44.5), 91 (66-130.75), 110 (79-138.8), and 149.5 (120-199.75) respectively. There was evidence of dyslipidemia (22.9%) including hypertriglyceridemia (5.7%), low HDL (22.9%), high LDL (2.9%), hypercholesterolemia (7.1%) with a p - value of 0.53, 0.05, 0.07, 0.57, 0.57 respectively in our DCMP patients (Table 4).
| Table 4: Laboratory investigations | ||||||
| Parameters | Male (n = 48) | Female (n = 22) | Total (n = 70) | |||
| Mean | SD | Mean | SD | Mean | SD | |
| Hb (mg/dl) | 11.9 | 2.9 | 10.8 | 1.7 | 11.6 | 2.6 |
| TLC (/mm3) | 9546.5 | 3690.2 | 9646.8 | 4686.1 | 9578 | 3995.1 |
| TPC (Lakh/mm3) | 2.4 | 1.1 | 2.0 | 0.6 | 2.2 | 0.9 |
| Bilirubin (mg/dl) | 1.1 | 0.8 | 1.1 | 0.9 | 1.1 | 0.8 |
| AST (IU/L) | 64.0 | 83.8 | 105.6 | 171.0 | 77.1 | 118.6 |
| ALT (IU/L) | 81.1 | 137.0 | 93.2 | 163.5 | 84.9 | 144.7 |
| Urea (mg/dl) | 49.5 | 30.5 | 66.8 | 57.1 | 54.9 | 41.2 |
| Creatinine (mg/dl) | 1.3 | 1.2 | 2.0 | 2.6 | 1.5 | 1.8 |
| RBS (mg/dl) | 135.9 | 71.9 | 172.4 | 94.9 | 147.4 | 80.9 |
| Serum Na (mmol/L) | 137.0 | 5.9 | 134.1 | 7.8 | 136.1 | 6.6 |
| Serum K (mmol/L) | 4.1 | 0.6 | 4.5 | 0.6 | 4.2 | 0.6 |
| Serum Albumin (g/dl) | 3.5 | 0.4 | 3.3 | 0.7 | 3.5 | 0.5 |
| FT3 | 3.2 | 1.1 | 3.1 | 0.6 | 3.1 | 0.9 |
| FT4 | 1.3 | 1.2 | 0.9 | 0.2 | 1.2 | 1.0 |
| TSH | 4.3 | 2.6 | 5.1 | 3.0 | 4.5 | 2.7 |
Mean RBS was 147.4 ± 80.9 mg/dl and median (IQR) RBS was 120 (103-157) with evidence of hypoglycemia in 8.6% of patients with a p - value of 0.42. Mean serum sodium and potassium levels were 136.1 ± 6.6 mmol/l and 4.2 ± 0.6 mmol/l respectively with hyponatremia and hypokalemia observed in 11.4% and 10% respectively. It was seen that hyponatremia was significantly correlated with ejection fraction (Correlation coefficient = (-) 0.25). A negative coefficient implied that patients with hyponatremia were seen to have lower ejection fraction levels. The results were significant (p = 0.0368) (Table 4).
Mean serum vitamin B12 levels were 373.13 ± 202.38 (male: 344.6 ± 148, female: 435 ± 282.17) pg/ml with a p - value of 0.08 and median (IQR) of vitamin B12 levels of 311 (289-367) pg/ml with vitamin B12 deficiency was observed in 17.1% of patients. Mean serum calcium values were 8.84 ± 0.77 (male: 8.87 ± 0.84, female: 8.78 ± 0.60) with a p - value of 0.65 with median (IQR) serum calcium of 8.9 (8.5-9.37) with hypocalcemia in 40% patients. Mean serum magnesium levels were 1.78 ± 0.23 (male: 1.78 ± 0.25, female: 1.79 ± 0.18) with a p - value of 0.84 and median (IQR) serum magnesium of 1.8 (1.6-1.9) with hypomagnesemia in 12.9% patients. Mean serum vitamin D levels were 16.81 ± 11.52 pg/ml with a p - value of 0.12 and median (IQR) vitamin D levels of 13.5 (11.42-17.37) pg/ml (Table 5).
| Table 5: Lab. investigations | ||||||
| Parameters | Male (n = 48) | Female (n = 22) | Total (n = 70) | |||
| Mean | SD | Mean | SD | Mean | SD | |
| Vitamin D | 17.0 | 8.25 | 16.39 | 16.84 | 16.81 | 11.52 |
| Vitamin B12 | 344.6 | 148 | 435 | 282.17 | 373.13 | 202.38 |
| Calcium | 8.87 | 0.84 | 8.78 | 0.60 | 8.84 | 0.77 |
| Magnesium | 1.78 | 0.25 | 1.79 | 0.18 | 1.78 | 0.23 |
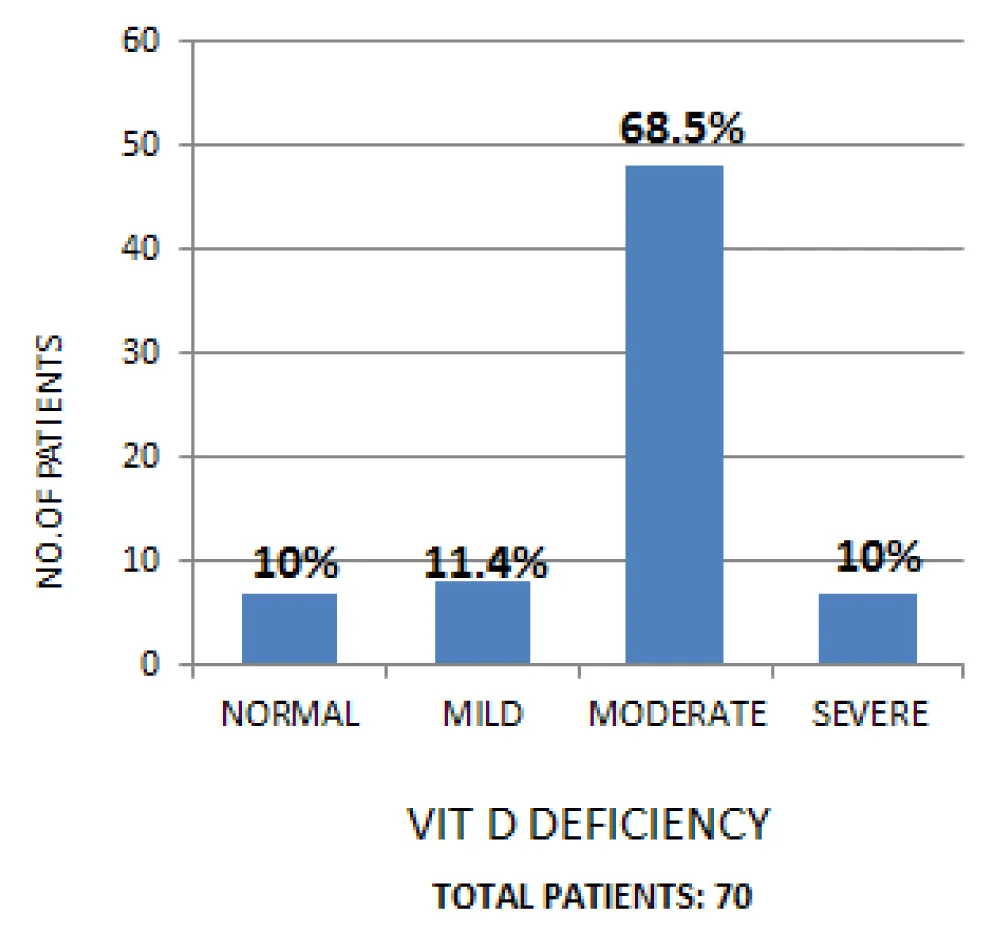
Out of 70 dcmp cases, 90% had vitamin D def.. with 78.5% patients had moderate-severe vitamin D deficiency (Figure 1 ). Vitamin D levels were correlated with ejection fraction and fractional shortening with a correlation coefficient of -0.102 and -0.105 respectively with a p - value of 0.40 and 0.38 respectively. A negative coefficient implied that patients with vitamin D def. were seen to have lower ejection fraction levels Table 6.
Figure 1: Spectrum of vitamin D deficiency in DCMP patients.
Table 6: Correlation b/w ECHO parameters and vit. D deficiency. |
||
| Ejection fraction (%) | Correlation Coefficient | -0.102 |
| p value | 0.402 | |
| N | 70 | |
| Fractional shortening (FS%) | Correlation Coefficient | -0.105 |
| p value | 0.385 | |
| N | 70 | |
| ESD (cm) | Correlation Coefficient | -0.063 |
| p value | 0.606 | |
| N | 70 | |
| EDD (cm) | Correlation Coefficient | -0.101 |
| p value | 0.404 | |
| N | 70 | |
A total of 70 patients who met the inclusion criteria were enrolled in the study. The data collected is non-parametric distributed.
Age and gender
Kumar M, et al. [3], a study of a total of 60 subjects observed that 66.7% were males while 33.3% were females (M:F:: 2:1) with a mean age of 48.37 ± 10.82 years. In a study by Kumar Divakar, et al. [8] of 30 DCMP patients, 56.6% were males and 43.25% were females with a mean age of 56.88 ± 15.99 years and 41.15 ± 20.19 years respectively. BoyillaVisalakshi, et al. [9], in a study of 60 DCMP cases observed that the majority of cases were above 60 years, comprised of 56.66% males & 43.33% females. Ushasree, et al. [10] in a study of 107 DCMP patients found that 71.96% of patients (adult) the ratio of adults male: female (M: F::2:1). Priya S, et al. [11] study of 116 subjects (56 DCMP patients vs. 60 controls), observed that mean age of DCMP patients was 48.6 ± 1.7 years. Polat Veil, et al. [12] study of 74 subjects (39 DCMP patients vs. 35 healthy subjects) observed 61.5% male and 38.5% female DCMP patients with a mean age of 50.4 ± 11.7 years.
Present study observations were consistent with the above-mentioned studies with peak incidence (30%) of DCMP patients seen in the age group 41-50 years age group with a mean age of 48.3 ± 15.2 years and 48 (69%) were males while 22 (31%) were females, out of 70 dcmp patients.
Clinical features
Kumar Divakar, et al. [8] study included 30 DCMP patients, having dyspnea (exertional) present in all DCMP patients (100%). NYHA IV dyspnea predominates with 46.6%, followed by NYHA III (33.3%), II (16.6%), and I (3.3%). Other symptoms were easy fatiguability (83.3%), pedal edema (70%), cough, palpitation, orthopnea, chest pain, abdominal pain, and syncope, present in DCMP patients. Kumar M, et al. [3] study of 30 DCMP studies demonstrated that there were 15 patients (50%) in NHYA class I, 14 patients in NHYA class II, and one patient in NHYA class III. Boyilla Visalakshi [9], et al. study of 60 DCMP patients observed that exertional dyspnea was present in all patients, followed by fatiguability (83.3%), pedal edema (70%), palpitation (56.6%), orthopnea (53.3%), PND (46.7%), chest pain (40%), syncope (16.6%). Massumi AR, et al. [13] study of 50 patients observed that dyspnea was seen in most of the patients (96%), followed by orthopnea (86%), edema (86%), fatigue (64%), chest pain (52%), palpitation (50%). Vanamali R.D, et al. [14] study of 37 DCMP patients observed that dyspnea (70%) was the predominant presenting complaint.
The current study findings were consistent with the above-mentioned studies as all patients were symptomatic i.e. having dyspnea (100%) with maximum patients having NYHA class IV (80%) followed by NYHA III (14.3%) class of dyspnea.
ECHO
Kumar Mukul, et al. [3] study of 30 DCMP studies demonstrated a mean LVEF of 30.47% having a significant difference from healthy controls of the same study. In a study, Kumar Divakar, et al. [8] of 30 DCMP patients reported mean LVEF as 30.87%. 40% of patients had EF of 20% - 29%, 36.6% had EF of 30% - 39%, 16.6% had EF of 40% - 45%, and 6.6% had EF of less than 20%. In a study of 60 DCMP cases by Boyilla Visalakshi, et al. [9] they demonstrated a mean LVEF of 30.87%. Sonowal N, et al. [14] in a study of 37 DCMP patients in N. East India observed a mean LVEF of 29.50% with a mean fractional shortening ((FS) of 8.08% in their patients. The current study findings were also consistent with the above-mentioned studies with mean LVEF of 26.6 ± 7.3% and mean % FS of 17.6% ± 3.1% with more than half of patients having E.F. of < 30%, and % F.S. between 15% - 19.9%.
Vitamin D
Vitamin D regulates numerous processes involved in the pathogenesis of HF, such as cell proliferation and differentiation, apoptosis, oxidative stress, inflammation, endothelial function, vascular calcification, and activation of the renin-angiotensin system. Similarly, vitamin D plays a critical role in heart failure pathophysiology in dilated cardiomyopathy via the presence of vitamin D receptors and vitamin D-dependent calcium binding protein in cardiac musculature including endothelium, vascular and cardiac myocytes, thereby possessing an antihypertrophic effect on cardiomyocytes. Moreover, 1,25(OH)2VitD3 also activates voltage-gated cardiac calcium channels, directly affecting cardiac contractile function or LV dimension, which can contribute to systolic dysfunction (i.e., reduced ejection fraction) in dilated cardiomyopathy [12]. Vitamin D is associated with functional status, illness severity, and prognosis in HF. This same study demonstrated a positive association between serum 25(OH)vitD3 levels (mean of 24.1 ± 10.4 ng/ml) and LV dimensions (i.e. mean LVEF of 27.6 ± 5.7%, mean fractional shortening of 13.1 ± 2.9) in dcmp patients and significant positive response was observed in terms of improvement in clinical status(NYHA class) and LVEF with vitamin D supplementation in vitamin D deficient heart failure patients [12].
Priya S, et al. [11] study found 24 out of 56 DCMP cases with severe Vitamin D deficiency (mean vitamin D levels, 14.5 ± 7.4 pg/ml). Vitamin D levels were negatively correlated with NT-proBNP, NYHA classification, and impaired LV systolic and diastolic function After correction for cardiovascular risk factors, the hazard ratio for death due to heart failure was significantly higher when Vitamin D deficient patients with 25(OH) Vitamin D levels < 10 pg/ml were compared with replete patients with levels > 30 ng/ml. Polat Veil, et al. [12] study demonstrated a positive association between serum 25(OH) Vitamin D levels (mean of 24.1 ± 10.4 pg/ml) and LV dimensions (i.e. mean LVEF of 27.6% ± 5.7%, mean fractional shortening of 13.1 ± 2.9) in DCMP patients. The current study is also consistent with the role of Vitamin D def. in dcmp as Vitamin D deficiency was observed in 90% of patients with a negative correlation coefficient b/w Vitamin D levels and EF levels.
Hypertension was observed in 10%, diabetes in 14.3%, history of alcohol intake (noncirrhotic dose) in 4.3%, and family history of dilated cardiomyopathy (DCMP) in 2.9% of patients as comorbidities. Vitamin D deficiency was observed in 90% of patients of which 78% had moderate to severe deficiency. Vitamin D could be the cause of DCMP which need to be corrected and follow-up of these cases should be done to assess the reversibility. Also in the current study, vitamin D levels were inversely correlated with the severity of heart disease in DCMP patients.
Limitations
No. of cases included in this study are less to have better statistical correlation and generalization of data i.e., needed more sample size. Follow-up of cases could not be done to assess the reversibility of etiologies of DCMP, as it was not part of this study.
- Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, Mockrin SC, Reinlib L. Report of the National Heart, Lung, and Blood Institute Special Emphasis Panel on Heart Failure Research. Circulation. 1997 Feb 18;95(4):766-70. doi: 10.1161/01.cir.95.4.766. PMID: 9054723.
- Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J. 1980 Dec;44(6):672-3. doi: 10.1136/hrt.44.6.672. PMID: 7459150; PMCID: PMC482464.
- Kumar M, Sharma Y, Bahl A. Comparative Analysis of Clinical Profile of Patients Admitted with Idiopathic Dilated Cardiomyopathy in a Tertiary Care Hospital. J Cardiovasc. Dis. Res. 2017; 8(2): 38-41.
- Masarone D, Kaski JP, Pacileo G, Elliott PM, Bossone E, Day SM, Limongelli G. Epidemiology and Clinical Aspects of Genetic Cardiomyopathies. Heart Fail Clin. 2018 Apr;14(2):119-128. doi: 10.1016/j.hfc.2017.12.007. PMID: 29525641.
- Arbustini E, Narula N, Tavazzi L, Serio A, Grasso M, Favalli V, Bellazzi R, Tajik JA, Bonow RO, Fuster V, Narula J. The MOGE(S) classification of cardiomyopathy for clinicians. J Am Coll Cardiol. 2014 Jul 22;64(3):304-18. doi: 10.1016/j.jacc.2014.05.027. Erratum in: J Am Coll Cardiol. 2014 Sep 16;64(11):1186. Bonow, Robert D [Corrected to Bonow, Robert O]. PMID: 25034069.
- Sanyal D, Raychaudhuri M. Infants with dilated cardiomyopathy and hypocalcemia. Indian J Endocrinol Metab. 2013 Oct;17(Suppl 1):S221-3. doi: 10.4103/2230-8210.119578. PMID: 24251165; PMCID: PMC3830311.
- Karabayir N, Kelesoglu E, Bornau H, Oztarhan K, Aktas D. Dilated Cardiomyopathy due to Vitamin D Deficiency. Int J Clin Cardiol. 2016; 3: 084.
- Vidyapati KD, Prasad ML, Manojet K. An Etiological Study of Dilated Cardiomyopathy in Correlation with Clinical, ECG and Echocardiographic profile. IOSR-JDMS May. 2017; 16: 5; 32-36.
- Boyilla V, Mogarala RR. A Study of Clinical Profile of Dilated Cardiomyopathy in Correlation with ECG and Echocardiography. IOSR-JDMS. 2019; 18: 3; 12:56-60.
- Ushasree B, Shivani V, Venkateshwari A, Jain RK, Narsimhan C, Nallari P. Epidemiology and genetics of dilated cardiomyopathy in the Indian context. Indian J Med Sci. 2009 Jul;63(7):288-96. PMID: 19700909.
- Priya S, Siddiqi Z, Karoli R, Fatima J, Gupta S, Mishra R. Study of Vitamin D Status in Patients with Dilated Cardiomyopathy at a Teaching Hospital in North India. J Cardiovasc Echogr. 2016 Jul-Sep;26(3):89-93. doi: 10.4103/2211-4122.187959. PMID: 28465969; PMCID: PMC5224667.
- Polat V, Bozcali E, Uygun T, Opan S, Karakaya O. Low vitamin D status associated with dilated cardiomyopathy. Int J Clin Exp Med. 2015 Jan 15;8(1):1356-62. PMID: 25785137; PMCID: PMC4358592.
- Massumi RA, Rios JC, Gooch AS, Nutter D, Devita VT, Datlow DW. Primary myocardial disease: report of fifty cases and review of the subject. Circulation. 1965 Jan;31:19-41. doi: 10.1161/01.cir.31.1.19. PMID: 14247526.
- Sonowal N, Rao VD. Clinical Profile of Patients with Dilated Cardiomyopathy in a Tertiary Care Center in North East India. J Evol Med Dent Sci. 2014; 3:30; 8378-8386. DOI: 10.14260/jemds/2014/3056.