More Information
Submitted: July 05, 2023 | Approved: July 31, 2023 | Published: August 01, 2023
How to cite this article: Raja Ramesh N, Ramesh D, Ramesh Babu P. Transcatheter Aortic Valve Implantation in Two High-Risk Patients with Low Coronary Ostial Heights Using the Novel Balloon-Expandable Myval Valve. J Cardiol Cardiovasc Med. 2023; 8: 089-099.
DOI: 10.29328/journal.jccm.1001159
Copyright License: © 2023 Raja Ramesh N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Balloon-expandable valves; Coronary artery obstruction; Coronary protection; Heart team; Myval; Transcatheter aortic valve implantation; Valve in valve
Transcatheter Aortic Valve Implantation in Two High-Risk Patients with Low Coronary Ostial Heights Using the Novel Balloon-Expandable Myval Valve
Raja Ramesh N1*, Ramesh Daggubati2 and Ramesh Babu P3
1Interventional Cardiologist, Aster Ramesh Hospitals, Vijayawada, India
2Associate Chief of Cardiology and Director of Structural Heart Disease, West Virginia University School of Medicine, United States
3Chief Cardiologist and Managing Director, Aster Ramesh Hospitals, Vijayawada, India
*Address for Correspondence: Dr. Raja Ramesh N, Interventional Cardiologist, Aster Ramesh Hospitals, Vijayawada, India, Email ID: [email protected]
The treatment of severe aortic stenosis by transcatheter aortic valve implantation (TAVI) is challenging in patients with high-risk coronary anatomy that is predisposed to iatrogenic or delayed coronary obstruction. Hence, the evidence on performing TAVI with adequate coronary protection with or without deploying a stent needs to be accumulated. We report two cases of TAVI performed in patients with low coronary heights, wherein a “wire only” strategy was used to provide coronary protection along with the implantation of a novel balloon-expandable Myval THV. The first patient underwent a valve-in-valve TAVI, while the second patient underwent the replacement of a native bicuspid Type 1A valve. This case series presents two high-risk TAVI cases wherein a guide extension catheter and a supportive coronary guidewire provided sufficient coronary protection. None of the cases required any rescue revascularization and no incidences of a new pacemaker implantation were reported.
Transcatheter aortic valve implantation (TAVI) has become a preferred choice for severe aortic stenosis (AS) and according to the recent 2020 American College of Cardiology/American Heart Association guidelines, TAVI is recommended as an effective alternative to surgical aortic valve replacement (SAVR) for suitable patients following heart team assessment, regardless of the surgical risk [1,2]. As the indication for TAVI expanded to lower- and intermediate-surgical-risk groups, the volumes of TAVI for severe AS have increased tremendously [3]. This progress is coupled with the sophisticated design advancements in transcatheter heart valves (THVs), catheter delivery systems, and vascular closure devices, which have resulted in improved clinical outcomes of TAVI over the years [4]. Furthermore, with the evolution in THVs over time, multiple iterations of THV designs have become available, which incorporate better features to prevent adverse events, including paravalvular leakage, acute coronary obstruction, vascular injury, conduction disturbances, or new permanent pacemaker implantations [4].
One anatomical factor affecting the TAVI outcomes including mortality is the presence of low coronary ostial heights. Precisely, the following aortic root characteristics increase the risk for coronary obstruction: coronary ostial height < 11 mm from the annulus, narrow sinuses (diameter < 30 mm), long calcified native aortic leaflets, and valve-to-coronary distance (VTC) < 4 mm during valve-in-valve (ViV) procedures [5]. Coronary artery obstruction (CAO) may occur because of the displacement of the leaflets of either the native or the implanted aortic valve (AV), which can become fatal minutes after TAVI or during the 30-day postprocedural period [6]. Since this is one of the leading causes of 30-day mortality following TAVI, several research groups investigated the outcomes of TAVI in high-risk patients with smaller coronary anatomies [7] and described specific procedures to avert the risk of CAO in specific patient groups [8].
In the Karlsruhe registry, Conzelmann, et al. compared TAVI patients receiving either ACURATE Neo, CoreValve, Lotus, Portico, or SAPIEN XT/S3 THVs, in whom the average distance between the coronary and annulus planes was 6.4 mm ± 1.1 mm. Coronary obstruction occurred in 3.5% of patients and intraoperative death in 1.1% of patients [9]. In addition, no coronary obstruction was reported in 24.4% of patients who received the BE SAPIEN XT or S3 THVs and the patient population of this registry included 68.6% (n = 59) women [9]. A meta-analysis conducted by Akinseye, et al. on the outcomes of patients undergoing TAVR showed a 78% prevalence of implanting BE THVs. Yamamoto, et al. investigated the outcomes of performing pre-emptive coronary protection in patients having high-risk aortic root anatomical characteristics indicated for TAVR. Their study predominantly comprised women (70.4%), and the patients had short stature and a lower average BMI (22.2 kg/m2) than that observed in Western populations. Furthermore, the 1.5% acute coronary obstruction events (n = 10) reported in the study occurred majorly in women (70%) [10]. Even the meta-analysis by Akinseye, et al. on 40 studies reporting 96 cases of coronary occlusion showed that 81% of the TAVI patients at risk for coronary obstruction were women of advanced age (> 70 years) (Akinseye, et al. 2010). In an earlier clinical study of a total of 269 participants with a mean coronary height of 8.9 mm ± 1.2 mm who underwent TAVR, low coronary heights were reported among 10.8% of patients, of whom 24 patients (82.8%) were women (7). Hence, although not significantly pointed out in the literature, the female gender is a considerable factor predisposed to coronary occlusion during TAVR.
In the case of native valve replacements, pre-emptive coronary wiring has been suggested as an effective technique to protect the flow through the coronary ostia following TAVI. In cases of eventual coronary flow obstruction, the “chimney” stenting approach has shown efficacy in limiting the rates of cardiac death and reducing the risk of delayed coronary obstruction for up to three years [11]. Successful TAVI outcomes using this technique require scrupulous patient selection with multidetector-row computed tomography (CT) analysis of the aortic root, which is the most crucial step of preprocedural planning. It involves the insertion of a guide extension catheter followed by pre-dilatation using a therapeutic balloon to provide pre-emptive protection of the left main coronary artery (LMCA) (Abramowitz, et al. 2015) or other arteries at risk. The outcomes of this technique may improve further if a low-profile THV is implanted in a supra-annular fashion. Another technique characterized by leaflet laceration or splitting (BASILICA technique) is also being considered promising. The outcomes of these techniques have been well elucidated in the recent literature [7,8,11-14]. Since the risk of acute and delayed CAO is much higher in ViV cases, more than one coronary protection strategy may be necessary to avert the risk and percutaneous “chimney” stenting has been reported as feasible in various studies with large populations [8]. While these investigations have helped evolve the patient selection criteria for TAVI, the optimum strategy to prevent CAO in patients with low coronary heights requires real-world evaluation. The available TAVI devices including newer generation THV designs with a lower profile need to be evaluated to report useful evidence on managing patients having numerous unmodifiable risk factors for TAVI, such as low coronary heights, female gender, and smaller body size that are common for Asian populations [7].
The newly introduced balloon-expandable (BE) Myval THV has a lower frame height, internal and external polyethylene terephthalate (PET) skirting, and a hybrid honeycomb design with open cells in the upper half, which ensure that coronary ostial jailing is avoided. The internal and external PET skirting enables the THV to mitigate the risk of paravalvular leakage or residual regurgitation, particularly in the acute postprocedural phase. This THV was used in a recent case of salvage redo-TAVI in a patient whose ACURATE Neo THV degenerated [15]. The authors elucidated the case of successful placement of the Myval THV in an intra-annular fashion below the nadir of the previous THV leaflets and correction of the distances from the THV to the left and right coronary artery (RCA) along with preventing acute obstruction of the RCA. There was no paravalvular leakage or recurrence of aortic regurgitation reported at the patient’s 30-day echocardiographic follow-up, thus showing acceptable outcomes. Several investigators have reported the favorable 30-day and hemodynamic outcomes of implanting the BE Myval THV in patients with low, high, and intermediate risks. Marked improvements in the AV hemodynamics were observed, with a significant reduction of residual AR and paravalvular leakage [16,17]. Secondly, the size matrix of this THV includes conventional sizes (20 mm, 23 mm, 26 mm, 29 mm), intermediate (21.5 mm, 24.5 mm, 27.5 mm), and extra-large sizes (30.5 mm and 32 mm), which is expected to reduce the risk of oversizing or under-sizing considerably and reduce the incidence of patient-prosthesis mismatch [18].
In lieu of the complexity surrounding the management of this subset of patients by TAVI, we present our report on two cases of TAVI in patients with low coronary heights along with other risk factors. The first patient underwent a ViV TAVI while the second patient received a replacement of the native valve despite being morbidly obese and having a bicuspid type 1A AV. Hence, we account for the case details of TAVI performed with the BE Myval THV along with the coronary protection strategy for both these patients. We report successful postprocedural outcomes with no incidences of iatrogenic complications including coronary flow obstruction or the need for a new pacemaker implant.
Case 1
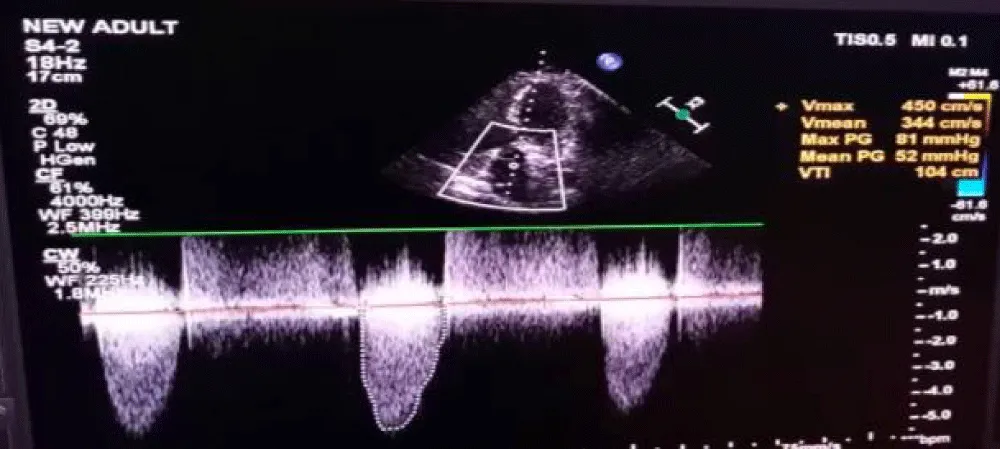
This is a case of a 70-year-old female with diabetes, hypertension, and severe AS status post-SAVR three years ago. In her current presentation, she experienced NYHA III symptoms including dyspnea, and on examination, an aortic systolic ejection murmur was heard. Her 2-D echocardiogram showed findings consistent with stenosis of the AV prosthesis with a mean gradient of 52 mmHg (Figure 1), suggestive of persisting severe AS.
Figure 1: Doppler across aortic valve showing Vmax of 4.5 m/sec and mean gradient of 52 mmHg.
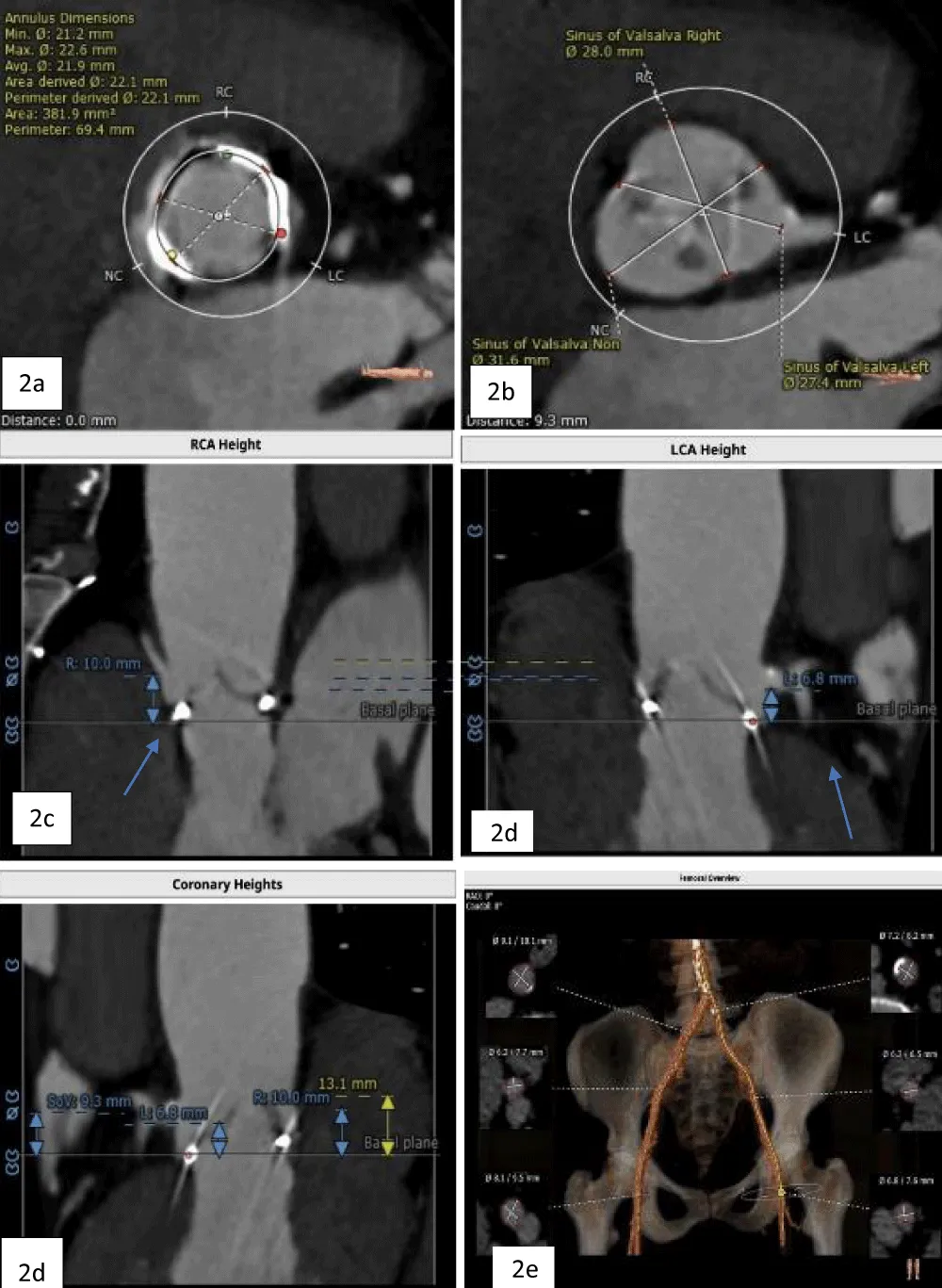
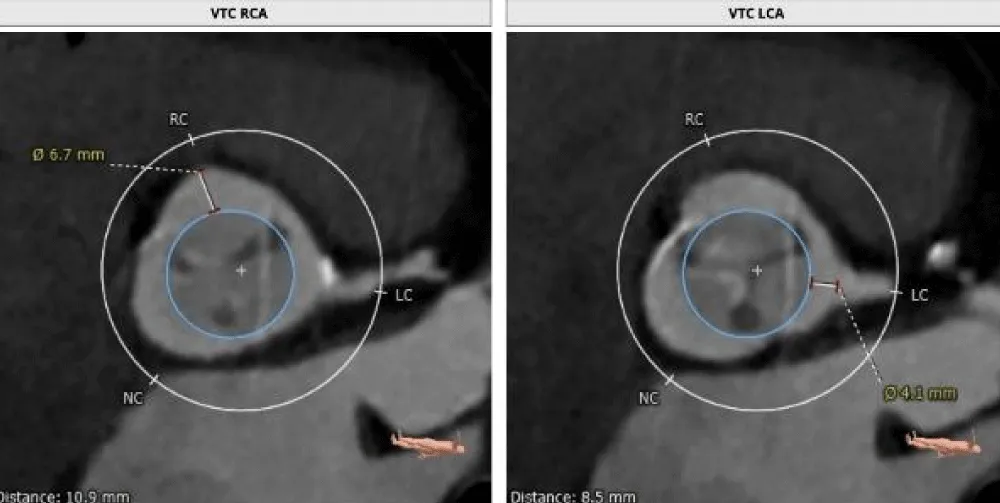
The patient’s past surgical notes were traced, which revealed that the initial AV replacement was performed using a 21 mm CROWN PRT™ stented bioprosthetic valve (LivaNova PLC, London, United Kingdom). Since the patient had high EuroSCORE II and STS scores, the Heart Team selected TAVI. Following the pre-TAVI screening, her coronary angiogram revealed non-obstructive lesions in the left anterior descending (LAD) artery. The 2-D contrast-enhanced CT and aortography revealed an average aortic annulus diameter of 21.9 mm, annular area of 381.9 mm2, and annulus perimeter of 69.4 mm, as seen in Figure 2a. The sinus of Valsalva (SOV) diameters of the left, right, and non-coronary cusps were 27.4 mm, 28 mm, and 31.6 mm, respectively (Figure 2b). However, the right and left coronary ostial (LCO) heights were 10 mm and 6.8 mm, respectively, both being abnormally low including the critically short LCO that was well visualized on CT (Figure 2c,2d). The SOV height was 9.3 mm and the height of the sino-tubular junction (STJ) was 13.1 mm (Figure 2e), while the average diameter of the STJ was 28.2 mm. In terms of access, both the femoral and iliac arteries had acceptable diameters on both sides without severe calcification showing suitability for a transfemoral approach (Figure 2f). On 2D-CT, the predicted VTC distance was 6.7 mm for the right coronary ostia (RCO) and 4.1 mm for the LCO, if a 21.5 mm Myval THV was used (Figure 3). The 21.5 mm BE Myval (Meril Life Sciences Pvt. Ltd., Vapi, India) was selected to be deployed under rapid pacing.
Figure 2: a. Annular dimensions b. SOV diameters of the left, right, and non-coronary cusps c. Heights of the LCA (6.8 mm) and RCA (10 mm) (blue arrows) d. Showing height of SOV (9.3 mm), and e. The diameters of the iliofemoral artery at the access site.
Figure 3: Showing the VTC reconstructed on 2D-CT (left: 6.7 mm; right: 4.1 mm) with the implantation of the selected 21.5 mm Myval THV.
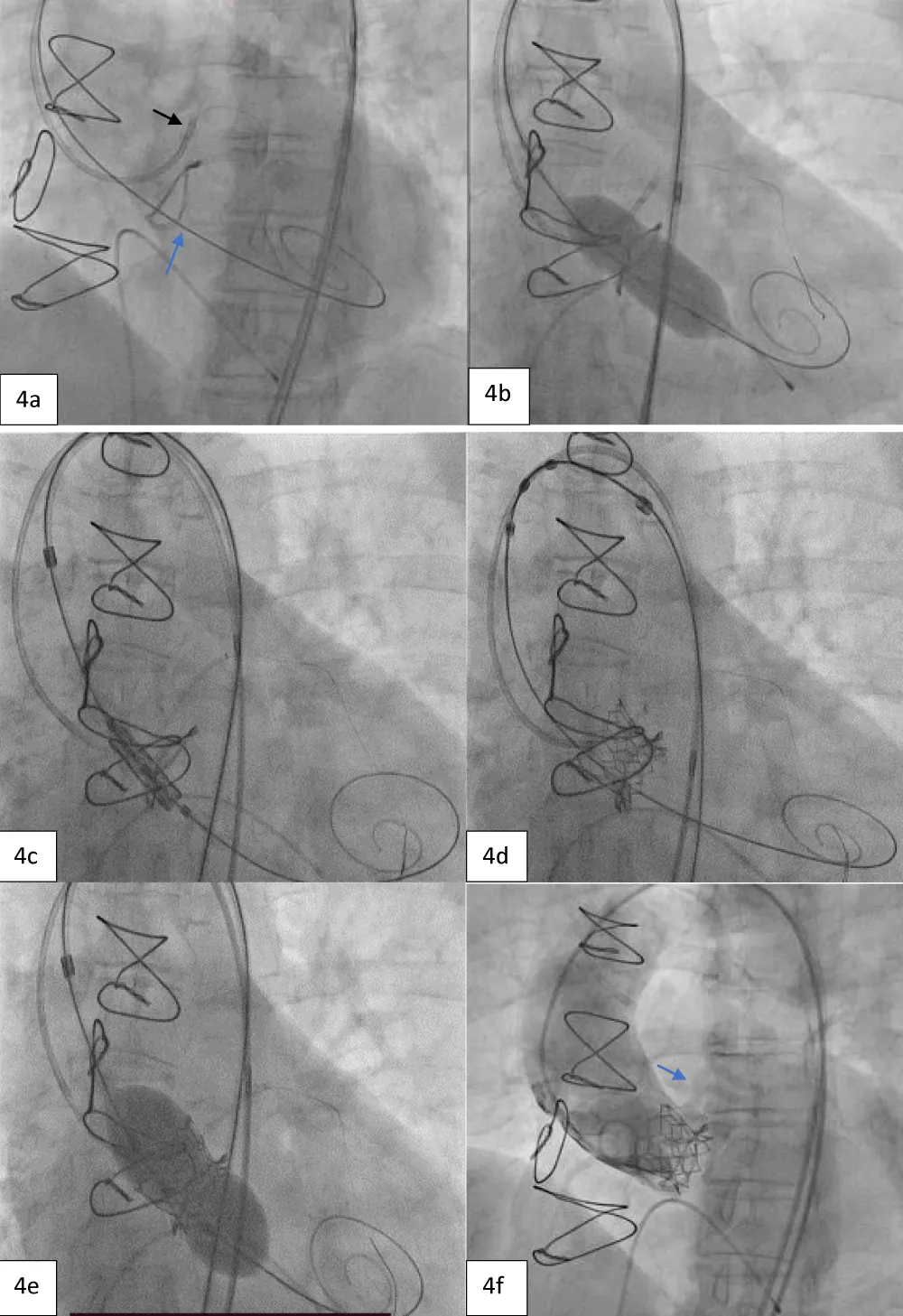
The TAVI was started by placing the patient under conscious sedation. An AL-1 catheter and a straight-tip Terumo wire (GLIDEWIRE® Standard, Terumo Corporation, Tokyo, Japan) were utilized to cross the valve. Subsequently, the AL-1 catheter was exchanged with a pigtail catheter, and a Safari extra support stiff guidewire (Boston Scientific Corporation, Marlborough, Massachusetts, USA) was placed at the LV apex for observing the periprocedural hemodynamics (i.e., the mean and peak pressure gradients). Then, a pacing lead wire was inserted into the right ventricle through the left femoral vein and a guiding catheter was inserted into the left CFA to protect the LCO (Figure 4a). The larger 14-Fr Python Introducer Sheath (Meril Life Sciences Pvt. Ltd., India) was advanced into the right CFA for THV delivery.
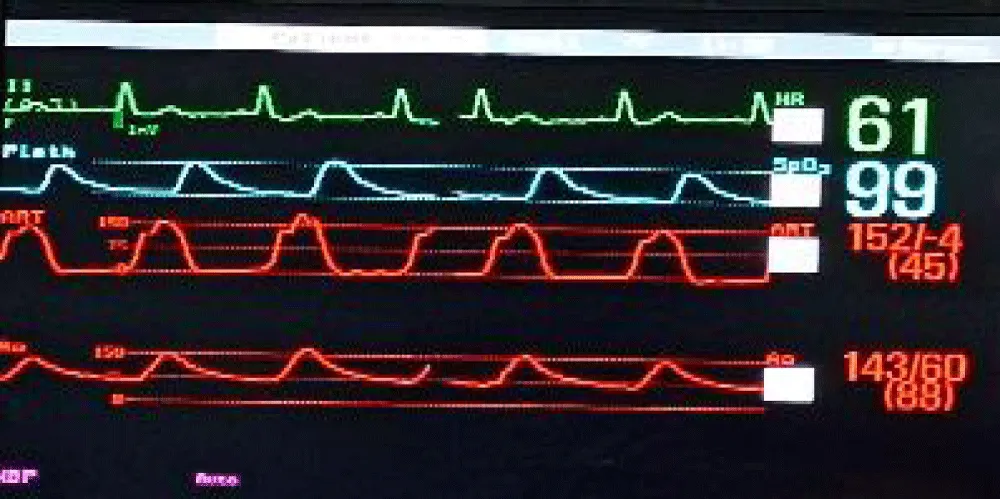
Next, we performed LMCA cannulation using a 6-Fr extra support backup guiding catheter (Medtronic, Minneapolis, USA) followed by placing a 180-cm long, 0.014” Runthrough® straight-tip coronary guidewire (Terumo Medical Corporation, Tokyo, Japan) through the LAD and placing a guide extension catheter in the LMCA (Guidezilla II; Boston Scientific Corporation, Marlborough, Massachusetts, USA) (Figure 4a). This ensured the adequate coronary protection of the LMCA. We proceeded with pre-dilatation for the deployment of the Myval THV using a 20 × 40 mm Mammoth™ balloon (Figure 4b) and deploying the 21.5 mm Myval THV under rapid pacing (Figure 4c,4d). After valve deployment, the patency of LMCA was evaluated by running an aortogram. One post-dilatation was performed using the same balloon by the addition of an extra 2 cm3 inflation volume (Figure 4e) followed by an evaluation of post-valve deployment patency of LMCA on the aortogram (Figure 4F). Upon confirming the final patency of the LMCA, the guide extension catheter from the LMCA and the guidewire from the LAD were gradually retracted. On the postprocedural echocardiogram, the residual peak-to-peak gradient across the left ventricle (LV) and aorta was 9 mmHg (Figure 5), and the mean and peak gradients were 8 mmHg and 8 mmHg. The procedure was completed by wound sealing at the right femoral site with one Proglide® and one Angio-seal® suture (St Jude Medical, St Paul, Minnesota, USA).
Figure 4: Showing protection of left main artery with guide extension catheter and guidewire (black arrow) and Safari stiff wire across the previous prosthetic valve (blue arrow). b. Pre-dilatation with 20x40 mm Mammoth balloon. c. Positioning of 21.5 mm Myval across the previous bioprosthetic valve. d. Deployment of the BE Myval THV with coronary protection in situ. e. Post dilatation performed using the same Mammoth over-the-wire balloon. f. Final aortic root angiogram showing patent LMCA (blue arrow).
Figure 5: Postprocedural 2D-echocardiogram of case 1 showing the pressure tracing of LV and aorta with the peak-to-peak gradient of 9 mmHg, indicating stable hemodynamics post TAVI.
Case 2
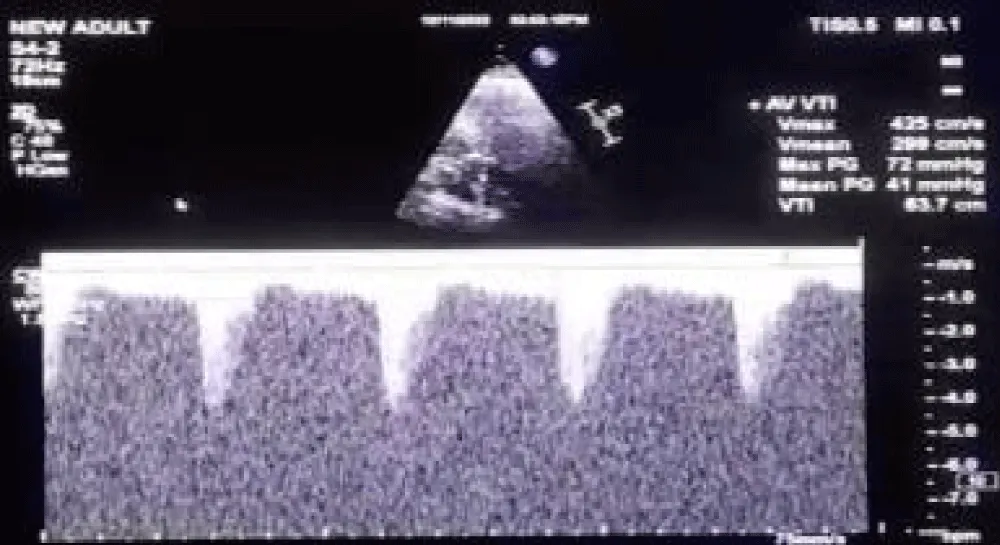
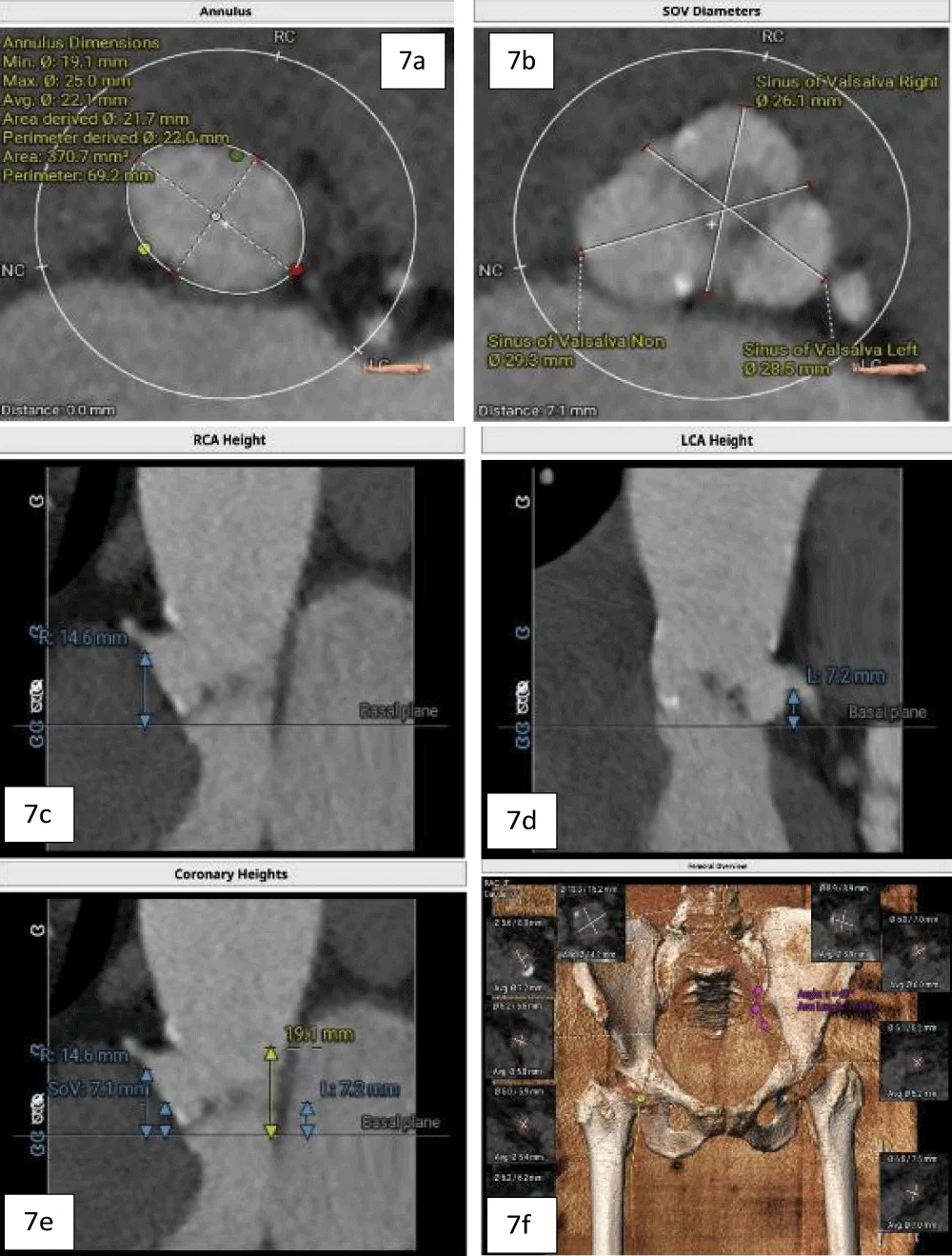
A 63-year-old female with morbid obesity (weight: 115 kg) presented with recurrent episodes of heart failure. On examination, an aortic systolic ejection murmur was audible, while the echocardiogram showed findings of severe AS with a mean gradient of 40 mmHg (Figure 6). Further, her coronary angiogram revealed non-obstructive CAD lesions in the right coronary and LAD arteries. The heart failure episodes were attributed due to severe AS; therefore, valve replacement was planned. The decision of the Heart Team was in favor of TAVI in view of the patient’s advanced age and morbid obesity. Hence, the anatomical eligibility for TAVI was assessed by contrast-enabled 3D computed tomography (CT) of the aortic root and the AV. The CT analysis revealed the bicuspid type 1a AV in the patient with fused right and left cusps. The average aortic annulus diameter was 22.1 mm, the annular area was 370.7 mm2, and the annulus perimeter was 69.2 mm (Figure 7a). The SOV diameters of the left, right, and non-coronary cusps are 28.5 mm, 26.1 mm, and 29.3 mm, respectively (Figure 7b). The heights of the RCO and LCO were 14.6 mm and 7.2 mm (Figure 7c,7d), respectively. The height of the SOV was 7.1 mm while the mean STJ height was 26.7 mm (Figure 7e). According to the iliofemoral analysis, the average diameter of the right common femoral artery (CFA) was 5.4 mm and that of the left CFA was 5.2 mm; the average diameter of the right external iliac artery (EIA) was 5.8 mm and that of the left EIA was 6.0 mm (Figure 7f).
Figure 6: Doppler echocardiogram of case 2 across the aortic valve showing Vmax of 4.25 m/sec, peak gradient of 72 mmHg, and the mean gradient of 41 mmHg.
Figure 7: a. Contrast-enhanced 2D-CT image showing the dimensions of the aortic valve annulus (mean annulus diameter: 22.1 mm and annulus perimeter: 69.2 mm), and b. showing the SOV diameters of the left, right, and non-coronary cusps. c. Height of the LCA (7.2 mm) shown with blue arrows, and d. Height of RCA (14.6 mm) shown with arrows. e. Height of the SOV 7.1 mm, shown with yellow arrows, and f. The diameters of the iliofemoral vessels at the access site.
Considering the morbid obesity, advanced age, the presence of a bicuspid Type 1a AV, and the low LCO height, the transfemoral TAVI with left-sided coronary protection was decided with the implantation of an available BE THV (Myval™) that is one of the newer-generation THVs having the lowest frame height [19].
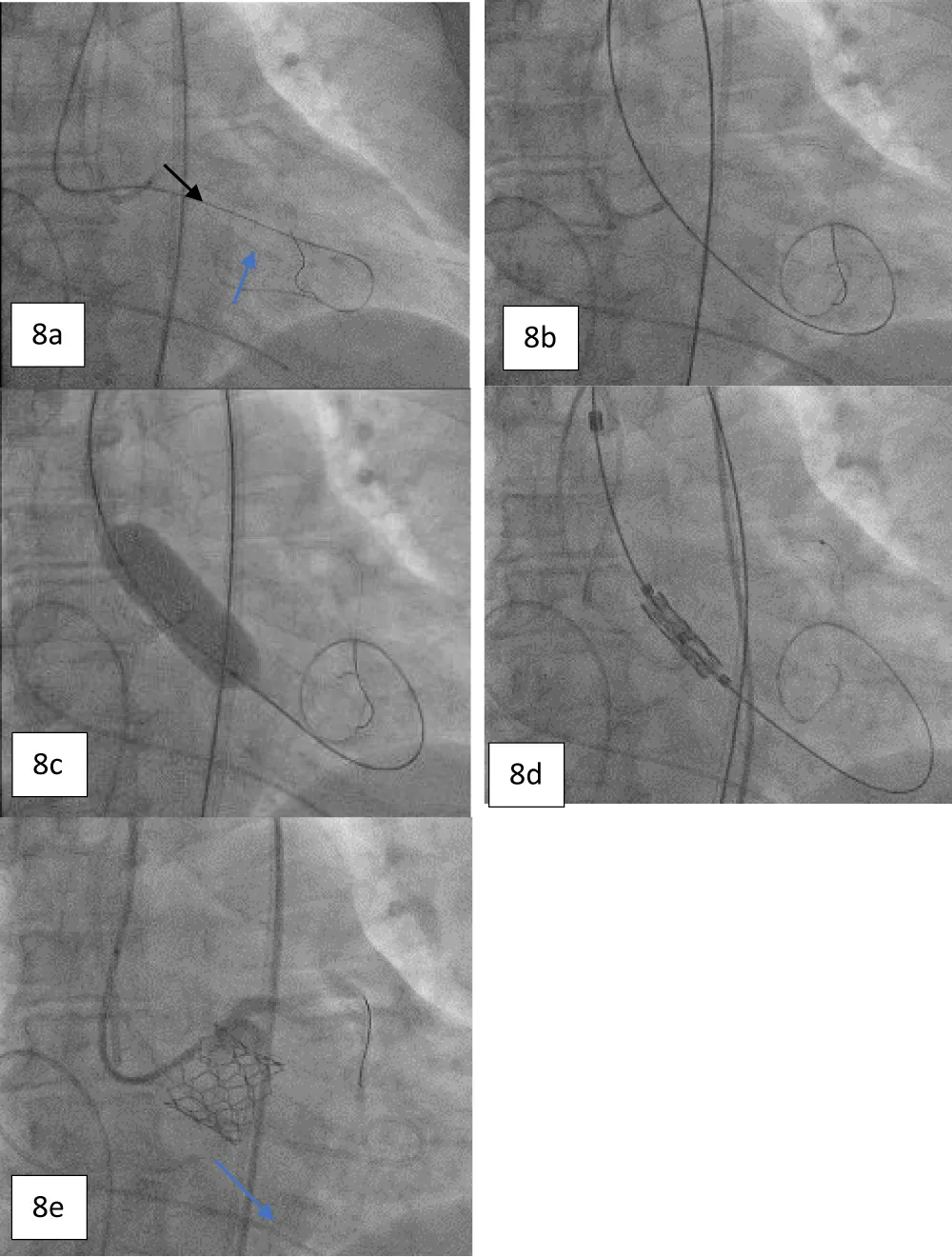
On the day of the procedure, after the patient registration process, the patient was taken to the cardiac catheterization laboratory, where she was placed under conscious sedation. The procedure was started by inserting a pigtail catheter through the left femoral artery and placing it at the non-coronary cusp of the aortic root to measure the AV hemodynamics. A temporary pacing lead wire was inserted through the left femoral vein and placed in the right ventricle. Thereafter, the LMCA was cannulated to provide coronary protection after introducing a coronary guiding catheter through the right radial artery with a straight-tip 0.014” Runthrough® coronary guidewire (Terumo Corporation, Tokyo, Japan) through a guide extension catheter (Guidezilla II; Boston Scientific Corporation, Marlborough, Massachusetts, USA) (Figure 8a-8e). The native AV was crossed using an AL-1 guiding catheter and a straight-tip guidewire (GLIDEWIRE® Standard, Terumo Corporation, Tokyo, Japan) was inserted. Thereafter, the AL-1 catheter was exchanged with a pigtail catheter and an extra-stiff supportive guidewire (Safari™; Boston Scientific Corporation, Marlborough, Massachusetts, USA) was placed in the LV apex. The 14-Fr Python Introducer sheath was advanced through the right femoral artery for the delivery of the selected 21.5 mm BE Myval valve. Pre-dilatation was performed using a 20 × 40 mm Mammoth™ over-the-wire balloon (Meril Life Sciences Pvt Ltd., Vapi, India) followed by the deployment of a 21.5 mm BE Myval THV under rapid pacing. Post-dilatation was performed by adding 1 cc extra volume to the 21.5 mm balloon of the Navigator™ THV delivery system (Meril Life Sciences Pvt Ltd., Vapi, India) that carried the crimped Myval THV. The postprocedural aortogram showed normal coronary flow and patent LMCA, while the postprocedural 2-D echocardiograph showed the mean and peak AV gradients of 10 mmHg and 18 mmHg, indicating stable hemodynamics without any aortic regurgitation or residual pressure gradient across the AV (Figure 9). Hence, the coronary guiding catheter was retracted. Both the femoral and the trans-radial access sites were closed with Perclose Proglide® suture-mediated foreclosure (Abbott Cardiovascular, Santa Clara, California, USA).
Figure 8: a. Angiogram showing the protection of the LMCA using a guide extension catheter and a supportive coronary guidewire (black arrow) with cannulation; crossing the diseased aortic valve using an AL-1 guiding catheter and a standard straight-tip wire (blue arrow). b. Guidewire exchange using a Safari stiff wire advanced across the valve. c. Pre-dilatation performed with a 20 x 40 mm Mammoth over-the-wire balloon. d. Positioning of 21.5 mm balloon-expandable Myval THV. e. Angiogram obtained post deployment showing the patent LMCA (blue arrow).
Figure 9: Postprocedural 2-D echocardiogram showing a simultaneous peak-to-peak pressure tracing of the LV and aorta with no residual gradient across the implanted aortic THV.
This series of two complex high-risk TAVI cases demonstrates the viability of the common coronary protection strategy employing a guide extension catheter into the LMCA with its cannulation performed prior to THV deployment and implanting a low-profile THV to replace the native or malfunctioning prosthetic aortic valve, despite that both the cases had markedly different risk factors for mortality post-TAVI. In the first case, ViV TAVI was performed successfully by using a protective guide extension catheter with an extra-stiff guidewire followed by pre-dilatation using the therapeutic over-the-wire balloon, Mammoth™. In this case, the THV was deployed above the previous prosthetic valve with the absence of coronary flow obstruction. Furthermore, the precise 2D-CT reconstructed analysis of the VTC distance, in this case, involving a ViV TAVI was a critical determinant of the risk for CAO in our experience. Since an intermediate size of 21.5 mm Myval THV was used, not only was THV under-sizing or oversizing avoided but the VTC was maintained above 4 mm as well, which was crucial for preventing CAO.
The postprocedural mortality rate associated with acute coronary occlusion increases to 40% - 50% in cases of ViV TAVI cases [8], while the risk of coronary flow obstruction in such cases is reported to be 2.3% [8,20]. Considering such a scenario, coronary protection of the LMCA is attempted prior to TAVI to prevent acute LMCA occlusion [21], which may necessitate immediate rescue revascularization. Using the guide extension catheter and implanting the Myval THV (having a relatively low frame height) ensured that the distance from the STJ to the bioprosthetic leaflet was not shortened spontaneously spontaneously because of the supra-annular placement of the Myval THV facilitated by the intraprocedural use of the radiopaque annular ring of the degenerated stented bioprosthesis as the precise location marker. Moreover, the placement of this newer-generation, lower-profile TAVI system is critically controlled against the minimal gap between the plane of the degenerated bioprosthesis to the STJ. In the past, improved survival rates have been reported when preventive coronary protection with the “wire only” technique was provided to TAVI patients having a high risk for coronary occlusion [10,11].
As regards the second case of an elderly woman with a bicuspid valve with fused right and left coronary cusps, a left-sided coronary protection strategy was utilized focusing on LMCA protection using a supportive guidewire for cannulation. Given that the female gender has been ascertained as a risk factor for coronary obstruction according to the findings of recent meta-analysis and large multicenter registries, the need for adequate coronary protection was heightened in this case [10,22]. The selection of the 21.5 mm Myval THV was appropriate since it had a short frame height of 18.35 mm, which caused no protrusion into the aortic root lumen, thus eliminating the need for chimney stenting or other coronary protection techniques. Also, both patients did not have any leaflet calcification, thereby ruling out the need for additional coronary protection by chimney stenting. In addition, the minimal infra-annular depth (≤ 3.5 mm) of the Myval THV helped avoid LVOT obstruction and mitigate the need for new pacemaker implantation. The selection of the Myval THV for TAVR was instrumental in both these cases, which allowed the TAVR operators to prevent many of the anticipated complications such as postprocedural paravalvular leakage and CAO. The low-profile design of the valve played a detrimental role in maintaining an optimal gap between the THV annular planes and the STJ, thus avoiding any sinus sequestration. Chimney stenting involves the positioning of an undeployed coronary stent above the newly implanted THV stent frame to offer coronary protection against flow occlusion. This becomes necessary in selected cases involving the implantation of self-expandable valves that have extended leaflet lengths, which may interfere with the anatomical relationships in the aortic root by inducing proximity to the STJ or obstructing the coronary cusps. The need for bailout chimney stenting may further arise if the long leaflets of the newly implanted THV obstruct the blood flow to the sinus of Valsalva leading to sinus sequestration or jailing, which did not occur in our cases.
In the case report by Casenghi, et al. TAVI-in-TAVI was performed on a patient with the previously implanted ACURATE neo L aortic valve (Boston Scientific Corporation, Marlborough, Massachusetts, USA). The patient had experienced cardiogenic shock and worsening infective endocarditis, which caused severe aortic regurgitation due to a leaflet tear. The authors remarked that the Myval THV had the lowest height among all other THVs, which played a crucial role in avoiding sinus sequestration, which could occur if the leaflets of the first implanted THV are pushed against the STJ. The Myval THV was implanted such that it was “completely below the nadir of the leaflets of the degenerated ACURATE neo.” Further, they report that the BE Myval THV (height: 18.85 mm) was implanted within the expanded ACURATE neo-stent frame (height: 19.5 mm). At one-month follow-up, the patient had no paravalvular leakage or symptoms of delayed CAO, and an overall improved clinical status (New York Heart Association class I).
The recently popularized BASILICA technique (Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic CAO during TAVI), has been investigated in large prospective, multicentre studies with successful outcomes reported based on VARC-2 endpoints, including 97.2% 30-day survival, 30-day stroke rate of 2.8%, and VARC-2 early safety of 82.8%. Overall, the registry showed BASILICA to be useful for ViV cases [8]. However, there is no consensus on the BASILICA technique for all ViV TAVI cases, as the decision to perform the technique is largely dependent on the patient selection criteria. Backer O and Søndergaard L [23] have pointed out that the BASILICA registry excluded patients having a high predisposition to CAO. Furthermore, the BASILICA technique did not eliminate the risk of CAO completely, as 10% of patients required emergent salvage coronary revascularization after undergoing BASILICA-TAVI and experiencing acute coronary obstruction). Hence, we believe that a non-complex coronary protection strategy involving a meticulous selection of supportive coronary guidewires and guiding catheters may have contributed to the procedural success in our cases.
Limitations
Although there was no selection bias in the study, this case series accounting for two patients having low coronary heights who underwent high-risk TAVI procedures is limited by its small size. However, it is noteworthy that the accessibility to sophisticated healthcare facilities is limited in low and middle-income countries such as India, where these cases are being reported. Further investigation is warranted with a larger study size to fully ascertain the clinical benefits of the coronary protection strategy utilized for these patients using the BE Myval THV.
In severe AS patients with high-risk coronary anatomy that is predisposed to acute or delayed coronary obstruction during TAVI, meticulous pre-procedural CT planning and adequate coronary protection are necessary to prevent catastrophic complications. Using the “wire only” strategy was beneficial in both these cases since it was combined with the implantation of a lower profile, newer generation BE Myval THV. Not only does this novel THV design incorporate a hybrid open structure that can accommodate larger circumscribable annular diameters, but its low frame height also avoids coronary ostial compromise as well. TAVI with the Myval THV was proven to be safe and effective in preventing acute coronary obstruction in this case series. Nevertheless, larger real-world studies should be conducted in the future to reaffirm these findings.
Ethics statement
The need for informed consent was waived by the institutional review board of the participating clinical centers as no identifiable information regarding the patients was used in the manuscript. The investigators of the study have adhered to the ethical principles as directed under the Declaration of Helsinki for the conduct of clinical research in human subjects.
Data availability statement
All details of the case presentations have been adequately described within the article. Requests for any additional information may be directly addressed to the corresponding author.
Financial disclosure: No financial support has been received from any non-profit or for-profit organization for the conduct of this case series.
- Sundt TM, Jneid H. Guideline Update on Indications for Transcatheter Aortic Valve Implantation Based on the 2020 American College of Cardiology/American Heart Association Guidelines for Management of Valvular Heart Disease. JAMA Cardiol. 2021 Sep 1;6(9):1088-1089. doi: 10.1001/jamacardio.2021.2534. PMID: 34287627.
- Writing Committee Members; Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021 Feb 2;77(4):450-500. doi: 10.1016/j.jacc.2020.11.035. Epub 2020 Dec 17. Erratum in: J Am Coll Cardiol. 2021 Mar 9;77(9):1276. PMID: 33342587.
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016 Apr 28;374(17):1609-20. doi: 10.1056/NEJMoa1514616. Epub 2016 Apr 2. PMID: 27040324.
- Santangelo G, Ielasi A, Pellicano M, Latib A, Tespili M, Donatelli F. An Update on New Generation Transcatheter Aortic Valves and Delivery Systems. J Clin Med. 2022 Jan 19;11(3):499. doi: 10.3390/jcm11030499. PMID: 35159952; PMCID: PMC8837046.
- Hsiung I, Spilias N, Bazarbashi N, Ahuja KR, Patel J, Kaur S, Rossi J, Gad M, Abdelfattah O, Saad A, Popovic Z, Miyasaka R, Yun J, Weiss A, Una S, Puri R, Reed G, Krishnaswamy A, Kapadia SR. Left main protection during transcatheter aortic valve replacement with a balloon-expandable valve. Journal of the Society for Cardiovascular Angiography & Interventions. 2022; 1(4): 100339. doi: 10.1016/j.jscai.2022.100339.
- Jabbour RJ, Tanaka A, Finkelstein A, Mack M, Tamburino C, Van Mieghem N, de Backer O, Testa L, Gatto P, Purita P, Rahhab Z, Veulemans V, Stundl A, Barbanti M, Nerla R, Sinning JM, Dvir D, Tarantini G, Szerlip M, Scholtz W, Scholtz S, Tchetche D, Castriota F, Butter C, Søndergaard L, Abdel-Wahab M, Sievert H, Alfieri O, Webb J, Rodés-Cabau J, Colombo A, Latib A. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018 Apr 10;71(14):1513-1524. doi: 10.1016/j.jacc.2018.01.066. PMID: 29622157.
- Nakashima M, Watanabe Y. Transcatheter Aortic Valve Implantation in Small Anatomy: Patient Selection and Technical Challenges. Interv Cardiol. 2018 May;13(2):66-68. doi: 10.15420/icr.2017:28:1. PMID: 29928310; PMCID: PMC5980651.
- Khan JM, Babaliaros VC, Greenbaum AB, Spies C, Daniels D, Depta JP, Oldemeyer JB, Whisenant B, McCabe JM, Muhammad KI, George I, Mahoney P, Lanz J, Laham RJ, Shah PB, Chhatriwalla A, Yazdani S, Hanzel G, Pershad A, Leonardi RA, Khalil R, Tang GHL, Herrmann HC, Agarwal S, Fail PS, Zhang M, Pop A, Lisko J, Perdoncin E, Koch RL, Ben-Dor I, Satler LF, Zhang C, Cohen JE, Lederman RJ, Waksman R, Rogers T. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: Results From the Multicenter International BASILICA Registry. JACC Cardiovasc Interv. 2021 May 10;14(9):941-948. doi: 10.1016/j.jcin.2021.02.035. Epub 2021 Mar 6. PMID: 33958168; PMCID: PMC8142224.
- Conzelmann LO, Würth A, Schymik G, Schröfel H, Anusic T, Temme S, Tzamalis P, Gerhardus J, Mukherjee C, Gonska BD, Schmitt C, Mehlhorn U. Feasibility of transcatheter aortic valve implantation in patients with coronary heights ≤7 mm: insights from the transcatheter aortic valve implantation Karlsruhe (TAVIK) registry. Eur J Cardiothorac Surg. 2018 Oct 1;54(4):752-761. doi: 10.1093/ejcts/ezy130. PMID: 29617804.
- Yamamoto M, Shimura T, Kano S, Kagase A, Kodama A, Koyama Y, Watanabe Y, Tada N, Takagi K, Araki M, Shirai S, Hayashida K. Impact of preparatory coronary protection in patients at high anatomical risk of acute coronary obstruction during transcatheter aortic valve implantation. Int J Cardiol. 2016 Aug 15;217:58-63. doi: 10.1016/j.ijcard.2016.04.185. Epub 2016 May 4. PMID: 27179209.
- Palmerini T, Chakravarty T, Saia F, Bruno AG, Bacchi-Reggiani ML, Marrozzini C, Patel C, Patel V, Testa L, Bedogni F, Ancona M, Montorfano M, Chieffo A, Olivares P, Bartorelli AL, Buscaglia A, Porto I, Nickenig G, Grube E, Sinning JM, De Carlo M, Petronio AS, Barbanti M, Tamburino C, Iadanza A, Burzotta F, Trani C, Fraccaro C, Tarantini G, Aranzulla TC, De Benedictis M, Pagnotta P, Stefanini GG, Miura M, Taramasso M, Kang JH, Kim HS, Codner P, Kornowski R, Pelliccia F, Vignali L, Taglieri N, Ghetti G, Leone A, Galiè N, Makkar R. Coronary Protection to Prevent Coronary Obstruction During TAVR: A Multicenter International Registry. JACC Cardiovasc Interv. 2020 Mar 23;13(6):739-747. doi: 10.1016/j.jcin.2019.11.024. Epub 2020 Feb 12. PMID: 32061608.
- Lederman RJ, Babaliaros VC, Rogers T, Khan JM, Kamioka N, Dvir D, Greenbaum AB. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: From Computed Tomography to BASILICA. JACC Cardiovasc Interv. 2019 Jul 8;12(13):1197-1216. doi: 10.1016/j.jcin.2019.04.052. PMID: 31272666; PMCID: PMC6724191.
- Carroll JD. The Splitting of Leaflets to Prevent Coronary Occlusion During TAVR. JACC Cardiovasc Interv. 2019 Jul 8;12(13):1253-1255. doi: 10.1016/j.jcin.2019.04.009. Epub 2019 Jun 12. PMID: 31202952.
- Khan JM, Greenbaum AB, Babaliaros VC, Rogers T, Eng MH, Paone G, Leshnower BG, Reisman M, Satler L, Waksman R, Chen MY, Stine AM, Tian X, Dvir D, Lederman RJ. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv. 2019 Jul 8;12(13):1240-1252. doi: 10.1016/j.jcin.2019.03.035. Epub 2019 Jun 12. PMID: 31202947; PMCID: PMC6669893.
- de Toledo JFB, Teixeirense PT, Guimaraes W, Motta J, Joaquim MR, Odone V, Mantovani J, Alves R, Gubolino LA. In-Series Transcatheter Aortic Valve Replacement-in-Transcatheter Aortic Valve Replacement: ACURATE neo-Transcatheter Heart Valve Degeneration Successfully Managed with Myval, Avoiding Coronary Flow Obstruction—A Case Report. Structural Heart. 2023. doi:10.1016/j.shj.2023.100181
- García-Gómez M, Delgado-Arana JR, Halim J, De Marco F, Trani C, Martin P, Won-Keun K, Montorfano M, den Heijer P, Bedogni F, Sardella G, IJsselmuiden AJJ, Campante Teles R, Aristizabal-Duque CH, Gordillo X, Santos-Martinez S, Barrero A, Gómez-Salvador I, Ancona M, Redondo A, Román JAS, Amat-Santos IJ. Next-generation balloon-expandable Myval transcatheter heart valve in low-risk aortic stenosis patients. Catheter Cardiovasc Interv. 2022 Feb;99(3):889-895. doi: 10.1002/ccd.29923. Epub 2021 Aug 14. PMID: 34390296.
- Delgado-Arana JR, Gordillo-Monge MX, Halim J, De Marco F, Trani C, Martin P, Infusino F, Ancona M, den Heijer P, Bedogni F, Nombela Franco L, Moreno R, Sargella G, Montorfano M, Aristizabal-Duque C, Romero-Delgado T, Santos S, Barrero A, Gomez Salvador I, IJsselmuiden S, Redondo Diéguez A, San Román Calvar JA, Amat-Santos IJ. Early clinical and haemodynamic matched comparison of balloon-expandable valves. Heart. 2022 May;108(9):725-732. doi: 10.1136/heartjnl-2021-319349. Epub 2021 Jul 20. PMID: 34285104.
- Abdelshafy M, Soliman O, Kim W, Ruck A, Elkoumy A, Elzomor H, Wang R, Tao L, Garg S, Mylotte D, Onuma Y, Serruys P. Quantitative angiographic assessment of aortic regurgitation posts 11 different types of TAVI devices a multicentre pooled analysis of 2665 valves. European Heart Journal. 2022; 43(2): ehac544-2078. doi: 10.1016/j.jscai.2022.100037
- Casenghi M, Oliva OA, Squillace M, Bellamoli M, Poletti E, Popolo Rubbio A, Testa L, Bedogni F, De Marco F. Bailout From Sinus Jailing: In-Series TAVR-in-TAVR to Avoid Coronary Flow Obstruction. JACC Case Rep. 2021 Apr 21;3(4):678-681. doi: 10.1016/j.jaccas.2021.02.022. PMID: 34317602; PMCID: PMC8302790.
- Ribeiro HB, Rodés-Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, Makkar R, Simonato M, Barbanti M, Schofer J, Bleiziffer S, Latib A, Hildick-Smith D, Presbitero P, Windecker S, Napodano M, Cerillo AG, Abdel-Wahab M, Tchetche D, Fiorina C, Sinning JM, Cohen MG, Guerrero ME, Whisenant B, Nietlispach F, Palma JH, Nombela-Franco L, de Weger A, Kass M, Sandoli de Brito F Jr, Lemos PA, Kornowski R, Webb J, Dvir D. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018 Feb 21;39(8):687-695. doi: 10.1093/eurheartj/ehx455. PMID: 29020413.
- Allende R, Barbosa Ribeiro H, Paradis JM, Doyle D, Pasian S, Rodés-Cabau J. Guidewire protection for a valve-in-valve transcatheter aortic valve implantation procedure with high-risk for coronary obstruction. Arch Cardiol Mex. 2014 Oct-Dec;84(4):322-4. doi: 10.1016/j.acmx.2014.04.005. Epub 2014 Sep 4. PMID: 25200591.
- Akinseye OA, Jha SK, Ibebuogu UN. Clinical outcomes of coronary occlusion following transcatheter aortic valve replacement: A systematic review. Cardiovasc Revasc Med. 2018 Mar;19(2):229-236. doi: 10.1016/j.carrev.2017.09.006. Epub 2017 Sep 12. PMID: 29102344.
- De Backer O, Søndergaard L. Is BASILICA the Standard for Preventing Coronary Obstruction in High-Risk Transcatheter Aortic Valve Replacement? JACC Cardiovasc Interv. 2021 May 10;14(9):949-951. doi: 10.1016/j.jcin.2021.03.028. PMID: 33958169.
- Ali N, Alnasser S, Latter D, Peterson MD, Fam NP. Double chimney stenting and triple kissing balloon inflation to prevent coronary obstruction during valve-in-valve TAVI with balloon valve fracture. EuroIntervention. 2022 Jul 22;18(4):e349-e350. doi: 10.4244/EIJ-D-22-00092. PMID: 35499840; PMCID: PMC9912954.