More Information
Submitted: September 11, 2023 | Approved: September 25, 2023 | Published: September 26, 2023
How to cite this article: Rauf S, Kumar T, Kumar V, Nath RK. Correlation between the Values of Immature Platelet Fraction and Mean Platelet Volume with the Extent of Coronary Artery Disease in Patients with Non-ST-Segment Elevation Myocardial Infarction. J Cardiol Cardiovasc Med. 2023; 8: 114-121.
DOI: 10.29328/journal.jccm.1001163
Copyright License: © 2023 Rauf S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Non-ST elevation myocardial infarction; Immature platelet fraction (IPF); Mean platelet volume (MPV); Left anterior descending artery (LAD)
Abbreviations: RP: Reticulated Platelet; IPF: Immature Platelet Fraction; MPV: Mean Platelet Volume; CAD: Coronary Artery Disease; NSTEMI: Non-ST-Segment Elevation Myocardial Infarction; NSTE-ACS: Non-ST Elevated Acute Coronary Syndrome; GRACE: Global Registry of Acute Coronary Events; ECG: Electrocardiogram; DM: Diabetes Mellites; HTN: Hypertension; SVD: Single Vessel Disease; DVD: Double Vessel Disease; TVD: Triple Vessel Disease; EF: Ejection Fraction; ROC: Reciever Operating Characteristic; AUROC: Area Under the ROC Curve
Correlation between the Values of Immature Platelet Fraction and Mean Platelet Volume with the Extent of Coronary Artery Disease in Patients with Non-ST-Segment Elevation Myocardial Infarction
Shadab Rauf1*, Tarun Kumar2, Vijay Kumar3 and Ranjit Kumar Nath4
1Cardiology, Senior Resident, ABVIMS and Dr. Ram Manohar Lohia Hospital, New Delhi, India
2Cardiology, Professor, ABVIMS and Dr. Ram Manohar Lohia Hospital, New Delhi, India
3Pathology, Professor, and Head of Department, ABVIMS, and Dr. Ram Manohar Lohia Hospital, New Delhi, India
4Cardiology, Professor and Head of Department, ABVIMS and Dr. Ram Manohar Lohia Hospital, New Delhi, India
*Address for Correspondence: Dr. Shadab Rauf, Cardiology, Senior Resident, ABVIMS and Dr Ram Manohar Lohia Hospital, New Delhi, India, Email: [email protected]
Introduction: The identification of new markers of thrombotic risk and early diagnosis of Non-ST-segment Elevation Myocardial Infarction (NSTEMI) could allow the optimization of the therapy and predict short and long-term prognosis.
Aims and objective: We aimed to assess the impact of Immature Platelet Fraction (IPF) and Mean Platelet Volume (MPV) levels on the extent of Coronary Artery Disease (CAD) in patients with NSTEMI undergoing coronary angiography.
Methods: This is a prospective observational study in which 100 subjects of Non-ST Elevation Myocardial Infarction were recruited. For the measurement of platelet count, IPF and MPV samples were analyzed by an automated hematology analyzer (Sysmex XN 1000). Patients were subjected to coronary angiography as per institutional protocol and the extent of coronary artery lesion was noted.
Result: A cutoff of MPV (fL) ≥ 10.6 can predict the involvement of the left anterior descending artery (LAD) with a sensitivity of 84%, and a specificity of 50%. With the cutoff of IPF (%) ≥ 2.4, it can even predict the type of disease i.e., Double Vessel Disease (DVD), or Triple Vessel Disease (TVD) with a sensitivity of 97%, and a specificity of 19%. Mean IPF values and MPV levels were significantly higher in patients with LAD involvement i.e., 4.40 ± 1.72% (p = 0.003) and 12.45 ± 1.88 (p = 0.030) respectively than in patients without involvement of LAD i.e., 2.78 ± 1.50% and 11.08 ± 2.19 respectively.
Conclusion: Immature platelet fraction and mean platelet volume were significantly associated with the involvement of the left anterior descending artery which was the most commonly involved vessel in patients with NSTEMI. Mean platelet volume was also associated with TVD which was statistically significant. MPV and IPF can be useful early independent hematologic markers to identify patients with a higher risk for significant CAD as they are readily available and inexpensive.
Coronary Artery Disease (CAD) is caused by atherosclerotic changes within the walls of coronary arteries which lead to impaired blood flow causing myocardial ischemia [1]. NSTEMI is defined as chest discomfort that is severe and has at least one feature: a) Occurs at rest (or with minimum exertion) lasting > 10 minutes, b) It is relatively of recent onset (i.e., within the prior 2 weeks, c) Crescendo pattern and an elevated troponin I/T [2]. The diagnosis and prediction of significant CAD in non-ST-elevation ACS (NSTE-ACS) are done by using cardiac biomarkers, electrocardiography, symptoms, and cardiac risk factors [3]. Only 14% to 20% of patients who have undergone cardiac catheterization were found to have normal or nonsignificant CAD [4].
Studies have found that platelets play a central role in the initiation and extension of thrombosis at the site of ruptured plaque in NSTE-ACS which has led to the development of interest in platelet function studies as a diagnostic role in NSTE-ACS. Platelet size and reactivity are studied with the help of Mean platelet volume (MPV) [5]. Studies have shown that in patients with myocardial infarction, an increase in MPV is associated with poor clinical outcome, impaired angiographic reperfusion [6], higher rates of restenosis after coronary angioplasty [7], and more residual coronary thrombus after fibrinolytic therapy [8]. Various studies showed that MPV can be used for early detection of ACS and risk stratification when other cardiac biomarkers are normal [9].
Reticulated Platelets (RP) are the fraction of young platelets that have been recently released from the bone marrow and have greater aggregating potential due to their larger size and protein synthesis capability [10]. Another point of concern is that raised RP levels have been correlated with impaired response, or “high on-treatment platelet reactivity”, to antiplatelet drugs, such as aspirin, clopidogrel [11], and even with third-generation thienopyridine, prasugrel [12].
CAD is diagnosed only after the first cardiovascular event has occurred, therefore, it is necessary to identify early markers of atherosclerosis that can allow better stratification of cardiovascular risk [13]. Immature Platelets Fraction (IPF) is a precise, cheap, and reproducible parameter that displays a correlation with the rate of RP and thus turnover of platelets [14]. Studies that are in favor of IPF have shown an increase in IPF within 24 hours of a cardiovascular event indicates a poor prognosis, in spite of normal cardiac biomarkers, and even if the patients did not meet the GRACE (Global Registry of Acute Coronary Events) score risk factor [4]. The identification of new markers of thrombotic risk and early diagnosis of NSTEMI could allow the optimization of the pharmacological therapy and predict short and long-term prognosis. Therefore, we aimed to assess the impact of MPV, IPF levels, and the extent of CAD in patients with NSTEMI undergoing coronary angiography.
The prospective observational study comprising 100 subjects of Non-ST Elevation Myocardial Infarction recruited from the emergency department of the Hospital were included in the study from 1st August 2021 to August 2022 after obtaining approval from the institutional ethics committee, Hospital, New Delhi with IRB number 580. All subjects were assessed by clinical examination, electrocardiography, troponin T/I, and echocardiography, and accordingly, patients were labeled as non-ST elevation myocardial infarction (NSTEMI). It was ensured that they fulfilled the inclusion/ exclusion criteria. After consent, they were included in the clinical study. Demographic data were noted. Immature Platelet Fraction (IPF) and Mean Platelet Volume (MPV) were estimated in all the subjects. Blood Sample was collected on the presentation from patients in the emergency or coronary care unit into tubes containing Ethylene Diamine Tetra Acetate (EDTA) who were diagnosed to have NSTEMI. For measurement of platelet count, IPF, and MPV, samples were analyzed by an automated hematology analyzer (Sysmex XN 1000). Patients were subjected to coronary angiography as per institutional protocol and the extent of coronary artery lesions was noted with respect to the number of vessels involved, percentage of lumen obstructed, type of vessel involved, and types of coronary artery lesion. The extent of coronary artery lesion was correlated with serum levels of MPV and IPF.
Inclusion criteria include NSTEMI patients 1) Chest discomfort which is severe and has at least one feature a) Occurs at rest (or with minimum exertion) lasting > 10 minutes, b) It is relatively of recent onset (i.e., within prior 2 weeks), c) Crescendo pattern and an elevated troponin I/T. (2) All age groups. Exclusion criteria include k/c/o myeloproliferative disorders and malignancy, prothrombotic disease (pulmonary embolus, ischemic stroke, arterial and venous thrombosis), sepsis, Thrombotic Thrombocytopenic Purpura (TTP) and idiopathic thrombocytopenic purpura.
Statistical analysis
All continuous variables have been expressed as mean ± standard deviation or median with an interquartile range as per the distribution of data. Categorical variables have been expressed as number and their respective percentage. The normality of the data was tested using the Shapiro-Wilk/Kolmogorov Smirnov test. Differences in binary and ordinal variables between two independent groups were analyzed by the exact chi-square test. Differences between two independent groups were assessed with the independent t-test or Mann-Whitney-U-test based on the normality of the data and in ≥3 independent groups using the ANOVA/ Kruskal-Wallis test and the ROC curve was extrapolated from the results obtained. All these statistics were accompanied by 95% Confidence Intervals (CI). All the reported p - values are two-sided and p - values < 0.05 were considered to indicate statistical significance. All data entries and statistical analyses were performed by using SPSS® Version 23.0 software.
We included 100 patients of NSTEMI, the following is the demographic profile of these patients as depicted in Table 1. 69 (69.0%) of the participants were male and 31 (31.0%) of the participants were female. The mean Age (Years) was 55.02 ± 9.53. 6 (6.0%) of the participants had Ages between 31 Years - 40 Years, 29 (29.0%) of the participants had Ages between 41 Years - 50 Years, 39 (39.0%) of the participants had Ages between 51 Years - 60 Years, 24 (24.0%) of the participants had Ages between 61- 70 Years while only 2 (2.0%) of the participants had Ages between 71 Years - 80 Years. 27 Among the entire study population, the risk factor of CAD seen most frequently was HTN which was 60 (60.0%) while 42 (42.0%) of the participants had DM, 45 (45.0%) of the participants had a family history of CAD while 56 (56.0%) of the participants had a history of smoking. Regarding the Electrocardiogram (ECG) changes, the majority of the patients had ST Depression which was 48 (48.0%) followed by T Wave Inversion which 41 (41.0%), 6 (6.0%) of the participants had NonSpecific Changes and only 5 (5.0%) of the participants had LB. The mean EF (%) was 43.30 ± 11.04, out of which the majority of the participants i.e., 39(39%) had EF between 40% - 49%.
| Table 1: Summary of Basic Details. | |
| Basic Details | Mean ± SD || Median (IQR) || Min-Max || Frequency (%) |
| Age (Years) | 55.02 ± 9.53 || 56.50 (48.00 - 61.25) || 31.00 - 80.00 |
| Age | |
| 31 - 40 Years | 6 (6.0%) |
| 41 - 50 Years | 29 (29.0%) |
| 51 - 60 Years | 39 (39.0%) |
| 61 - 70 Years | 24 (24.0%) |
| 71 - 80 Years | 2 (2.0%) |
| Gender | |
| Male | 69 (69.0%) |
| Female | 31 (31.0%) |
| HTN (Yes) | 60 (60.0%) |
| DM (Yes) | 42 (42.0%) |
| Cardiac Details | Mean ± SD || Median (IQR) || Min-Max || Frequency (%) |
| EF (%) | 43.30 ± 11.04 || 45.00 (35.00-50.00) || 15.00 - 60.00 |
| EF | |
| <40% | 27 (27.0%) |
| 40 - 49% | 39 (39.0%) |
| ≥50% | 34 (34.0%) |
| DM: Diabetes Mellites, HTN: Hypertension EF: Ejection Fraction. | |
The mean IPF (%) of the study population was 4.14 ± 1.79 while the mean MPV (fL) was 12.23 ± 1.99 as shown in Table 2.
| Table 2: Mean IPF, MPV, and Total Platelet Count. | |||
| Investigations | Mean ± SD | Median (IQR) | Min - Max |
| Platelet Count (x10³/mm³) | 216.63 ± 84.56 | 192.50 (160.00 - 247.25) | 96.0 - 586.0 |
| IPF (%) | 4.14 ± 1.79 | 3.65 (3.00 - 5.43) | 0.8 - 9.3 |
| MPV (fL) | 12.23 ± 1.99 | 12.10 (11.20 - 13.80) | 7.8 - 17.2 |
| IPF: Immature Platelet Fraction, MPV: Mean Platelet Volume. | |||
Table 3 represents the association between IPF and MPV with various parameters. Both MPV and IPF were significantly raised in patients with HTN with a p - value of 0.002 and 0.001 respectively. However, there was no such association with Diabetes Mellitus (DM). Both MPV and IPF values were found to be inversely related to Ejection Fraction (EF) but only IPF was found to be statistically significant. Both MPV and IPF also had an inverse relationship with platelet count and both were statistically significant. Only MPV was found to be associated with the type of disease with a significant p - value of 0.02 there was no significant association found with vessels involved or the extent of involvement of various vessels.
Table 3: Association between IPF (%) AND MPV (fL) with Parameters.
|
||||
| Parameters | IPF (%) | p - value | MPV (fL) | p - value |
| ≥50% | 3.41 ± 1.26 | 11.79 ± 1.69 | ||
| Platelet Count (x10³/mm³)*** | Correlation Coefficient (rho) = -0.47 | <0.001 | Correlation Coefficient (rho) = -0.3 | 0.003 |
| Type of Disease | 0.591 | 0.020 | ||
| Non-Critical | 3.08 ± 1.05 | 10.72 ± 2.14 | ||
| SVD | 4.06 ± 1.99 | 10.62 ± 1.84 | ||
| DVD | 4.02 ± 1.58 | 11.98 ± 2.07 | ||
| TVD | 4.44 ± 1.90 | 12.71 ± 1.88 | ||
| Involvement: LM | 0.102 | 0.159 | ||
| Yes | 5.82 ± 2.66 | 13.65 ± 1.84 | ||
| No | 4.07 ± 1.73 | 12.18 ± 1.98 | ||
| Involvement: LAD | 0.003 | 0.030 | ||
| Yes | 4.40 ± 1.72 | 12.45 ± 1.88 | ||
| No | 2.78 ± 1.50 | 11.08 ± 2.19 | ||
| Involvement: LCX | 0.111 | 0.653 | ||
| Yes | 3.99 ± 1.77 | 12.18 ± 1.99 | ||
| No | 4.51 ± 1.80 | 12.38 ± 2.01 | ||
| Involvement: RCA | 0.538 | 0.398 | ||
| Yes | 4.11 ± 1.71 | 12.36 ± 1.90 | ||
| No | 4.20 ± 1.96 | 11.98 ± 2.16 | ||
| Extent of Involvement: LM | 0.259 | 0.259 | ||
| <50% | 9.00 ± 0 | 16.00 ± 0 | ||
| 50-70% | 3.30 ± 0 | 14.20 ± 0 | ||
| >70% | 5.50 ± 2.12 | 12.20 ± 0.28 | ||
| Extent of Involvement: LAD | 0.940 | 0.216 | ||
| <50% | 4.33 ± 1.93 | 11.85 ± 2.13 | ||
| 50 - 70% | 4.28 ± 1.72 | 11.77 ± 1.90 | ||
| >70% | 4.44 ± 1.72 | 12.68 ± 1.81 | ||
| Extent of Involvement: LCX | 0.554 | 0.299 | ||
| <50% | 3.23 ± 0.37 | 11.10 ± 1.93 | ||
| 50 - 70% | 4.18 ± 2.93 | 12.64 ± 2.48 | ||
| >70% | 4.05 ± 1.75 | 12.24 ± 1.96 | ||
| Extent of Involvement: RCA | 0.099 | 0.484 | ||
| <50% | 3.94 ± 1.38 | 12.37 ± 1.56 | ||
| 50 - 70% | 2.94 ± 1.02 | 11.46 ± 2.20 | ||
| >70% | 4.33 ± 1.79 | 12.50 ± 1.91 | ||
| Type of Lesion: LM | - | - | ||
| A | - | - | ||
| B | - | - | ||
| C | 5.82 ± 2.66 | 13.65 ± 1.84 | ||
| Type of Lesion: LAD | 0.830 | 0.201 | ||
| A | 4.26 ± 1.77 | 11.95 ± 2.13 | ||
| B | 4.35 ± 1.51 | 12.27 ± 1.77 | ||
| C | 4.51 ± 1.81 | 12.79 ± 1.77 | ||
| Type of Lesion: LCX | 0.694 | 0.122 | ||
| A | 4.28 ± 2.46 | 11.42 ± 2.05 | ||
| B | 3.67 ± 1.77 | 12.88 ± 2.44 | ||
| C | 4.14 ± 1.58 | 12.23 ± 1.77 | ||
| Type of Lesion: RCA | 0.239 | 0.244 | ||
| A | 3.46 ± 1.38 | 11.59 ± 1.85 | ||
| B | 4.68 ± 1.47 | 12.56 ± 1.56 | ||
| C | 4.28 ± 1.80 | 12.60 ± 1.92 | ||
| SVD: Single Vessel Disease, DVD: Double Vessel Disease, TVD: Triple Vessel Disease, LM: Left Main, LAD: Left Anterior Descending Artery, LCX: Left Circumflex Artery, RCA: Right Coronary Artery. | ||||
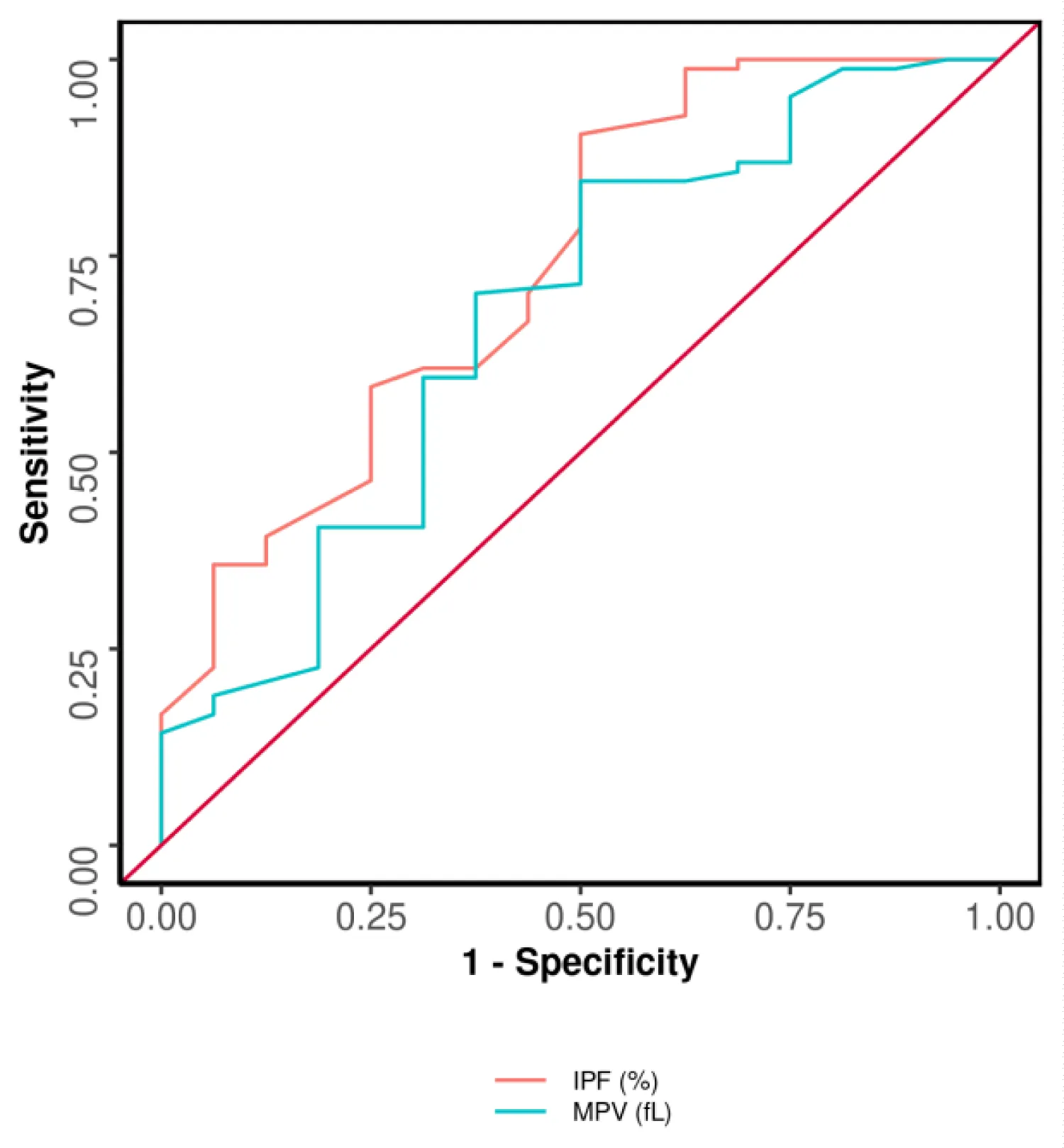
In Table 4 the area under the receiver operating characteristic curve (AUROC) for IPF (%) predicting involvement of LAD was 0.738 (95% CI: 0.595 - 0.882), thus demonstrating fair diagnostic performance which was statistically significant (p = 0.001), with a diagnostic accuracy of 84% and an odds ratio of 9.5. At a cutoff of IPF (%) ≥ 2.9, it predicts the Involvement of LAD with a sensitivity of 90% and a specificity of 50%.
| Table 4: Performance of Study Parameters for Predicting Involvement of LAD: Primary Diagnostic Parameters. | |||||||||
| Variable | Sensitivity | Specificity | PPV | NPV | Diagnostic Accuracy |
LR+ | LR- | Odds Ratio | p - value |
| IPF (%) (Cutoff: 2.9 by ROC) |
90.5% (82 - 96) | 50.0% (25 - 75) | 90.5% (82 - 96) | 50.0% (25 - 75) | 84.0% (75-91) | 1.81 (1.10 - 2.97) | 0.19 (0.08 - 0.43) | 9.50 (2.80 - 32.21) | < 0.001 |
| MPV (fL) (Cutoff: 10.6 by ROC) |
84.5% (75 - 91) | 50.0% (25 - 75) | 89.9% (81 - 96) | 38.1% (18 - 62) | 79.0% (70-87) | 1.69 (1.03 - 2.78) | 0.31 (0.15 - 0.62) | 5.46 (1.74 - 17.15) | 0.002 |
| PPV: Positive Predictive Value, NPV: Negative Predictive Value, LR: Likelihood Ratio, IPF: Immature Platelet Fraction, MPV: Mean Platelet Value, ROC: Receiver Operator Characteristics. | |||||||||
The AUROC for MPV (fL) predicting involvement of LAD was 0.672 (95% CI: 0.515 - 0.83) as seen in Table 4, thus demonstrating poor diagnostic performance. It was statistically significant (p = 0.002), with a diagnostic accuracy of 79% and an odds ratio of 5.46A cutoff of MPV (fL) ≥ 10.6 predicts the involvement of LAD with a sensitivity of 84% and a specificity of 50%.
The performance of Study Parameters for Predicting the Involvement of LAD is shown in Figure 1. The best parameter in terms of AUROC, sensitivity, negative predictive value, and diagnostic accuracy was found to be IPF (%). However, both MPV and IPF were found to be the best parameters in terms of specificity and positive predictive value.
Figure 1: Receiver operator characteristics curve analysis [ROC] showed diagnostic accuracy of IPF (%), and MPV (fL) in predicting the involvement of LAD. Trends: The best parameter in terms of AUROC: IPF (%). The best parameter in terms of sensitivity: IPF (%). The best parameter in terms of specificity: IPF (%), MPV (fL). The best parameter in terms of positive predictive value: IPF (%), MPV (fL). The best parameter in terms of negative predictive value: IPF (%). The best parameter in terms of diagnostic accuracy: IPF (%).
The major cause of mortality and morbidity in hospitals is CAD for which prompt diagnosis and appropriate treatment are essential. There is a need for markers for cardiac risk stratification and to identify patients who would most likely benefit from invasive cardiac studies [4,15].
Based on demographic characteristics of our study 69% were male which is similar to results obtained from Paramita, et al. in which males were 73.1% [16]. This is consistent with the theory that men are a separate risk factor for acute coronary syndrome. Based on age characteristics, it was found that patients with NSTEMI were predominantly in age group 41 years - 60 years (68%) out of which the majority (39%) lay among 51 years - 60 years of age which is similar to the study by Enna et al in which the average age of the patients was between 50 years - 59 years in NSTEMI/UAP patients [17]. The study conducted by Paramita, et al. [16] also found a relatively similar finding where 82.1% of the incidence of acute coronary syndrome was found in the young adult age group (aged 40 years - 60 years). In our study, 45% of cases had a family history of CAD, 40% of patients had hypertension, 58% had diabetes and 56% of the patients had a history of smoking. In a study by Rozi Khan et al in NSTEMI patients, DM was seen in 47.4% while HTN was seen in 46.5%, 36% had a positive smoking history, and a family history was seen in 16.3% [18]. Platelet activity has a diagnostic and predictive role in ACS as studies have shown that patients with CAD with increased immature platelets had worse clinical outcomes [19,20].
MPV is a commonly used marker for platelet size and a potential marker of platelet reactivity [5,21]. It is predictive of prognosis [21,22] percutaneous coronary intervention success rate, and reperfusion rate after thrombolytic therapy [23-25].
In our study, the mean (SD) IPF (%) was 4.14 ± 1.79, the median (IQR) IPF (%) was 3.65 (3 - 5.43) and the IPF (%) ranged from 0.8 - 9.3. The mean (SD) MPV (fL) was 12.23 ± 1.99, the median (IQR) MPV (fL) was 12.10 (11.2 - 13.8) and the MPV (fL) ranged from 7.8 - 17.2. This is also comparable to the study done by Erik, et al. [26] which also showed a significant increase in IPF in ACS patients. A study conducted by Michelle, et al. [27] 2015 showed that the mean IPF in ACS patients was 5 + 2.8 and there was no significant difference between STEMI and NSTEMI. A study done by Huang et al showed mean IPF in ACS patients was 3.7 + 2.64 and MPV (fL) was 10.7 + 0.80 [ 28]. Also study by Ahmad Hasim, et al. also showed that the mean value of MPV in NSTEMI was 9.22 ± 0.53 [29].
In our study, there was a moderate positive correlation between MPV (fL) and IPF (%) in NSTEMI patients and this correlation was statistically significant (rho = 0.41, p = 0.001). These results were also consistent with the study done by Pratia, et al. [16] which analyzed the correlation between MPV and IPF which also showed a positive correlation between IPF and MPV in ACS patients with p < 0.02, but the correlation was weak with r = 0.388. In our study levels of IPF and MPV were significantly higher in patients with HTN with a strength of association (Point-Biserial Correlation) of 0.3.
The mean (SD) of platelet count (x10³/mm³) was 216.63, the median (IQR) of platelet count (x10³/mm³) was 192.50 (160 - 247.25) and the platelet count (x10³/mm³) ranged from 96 - 586. There was a moderate negative correlation between platelet count (x10³/mm³) and IPF (%), and this correlation was statistically significant (rho = -0.47, p = < 0.001). Similar results were found in the study by Nardin, et al. [30] where patients with higher IPF displayed lower ejection fraction and lower platelet count. There was a weak negative correlation between platelet count (x10³/mm³) and MPV (fL), and this correlation was statistically significant (rho = -0.3, p = 0.003). These results are similar to the study by Taskesen, et al. where higher MPV was associated with low platelet count [31]. Blood samples were collected into tubes containing EDTA, it was seen that platelet count and its indices like IPF and MPV were rather stable over time with EDTA blood [32].
MPV is elevated in patients with hypertension as seen in the study by Li, et al. [33] and multiple clinical trials showed that higher MPV was associated with atherosclerotic conditions [34-38].
There was a moderate negative correlation between EF (%) and IPF (%), and this correlation was statistically significant (rho = -0.37, p = < 0.001) with the strength of Association (Kendall’s Tau) of 0.26 (Small Effect Size). The mean (SD) of platelet count (x10³/mm³) was 216.63 (84.56), the median (IQR) of platelet count (x10³/mm³) was 192.50 (160 - 247.25), and the platelet count (x10³/mm³) ranged from 96 - 586. There was a moderate negative correlation between platelet count (x10³/mm³) and IPF (%), and this correlation was statistically significant (rho = -0.47, p = < 0.001). Similar results were found in the study by Nardin, et al. [30] where patients with higher IPF displayed lower ejection fraction and lower platelet count. Yazici, et al. showed the correlation between left ventricular ejection fraction (LVEF) and MPV where they demonstrated lower MPV was associated with depressed LVEF (p = 0.02) [39] as MPV is associated with inflammation [40].
Our study showed that only 6% of patients had non-significant CAD which is similar to the result by Taskesen, et a.l in which 13% of patients with high troponin had nonsignificant CAD which may have been due to non-atherothrombotic causes of myocardial injury or noncardiac-causes of troponin elevation [31]. In our study 20.0% of the participants had single vessel disease (SVD), 34.0% had Double vessel disease (DVD) and 40.0% had Triple Vessel Disease (TVD). In a study by Arpan, et al., 34% had TVD (>50% luminal diameter stenosis), 28% had DVD, 26% had SVD, 13% had no significant stenosis, 10% had left main (LM) stenosis of > 50% [41]. Left main artery was involved in 4% of the cases, LAD was involved in 84% of the cases, LCX was involved in 71% of the cases and RCA was involved in 67% of the cases, similar results were seen in the study by Ahmed Seprham, et al. where LAD was the most commonly involved vessel with 58 .6% cases followed by LCX with 24.1% and RCA with 17.2% [42].
More than 70% luminal narrowing of the left main was seen in half of the cases and only one-fourth of cases had less than 50% of vessel involvement. However, 73.8% of cases had more than 70% involvement in LAD while 11.9% of cases had less than 50% involvement in LAD. 84.5% of cases with LCX involvement had more than 70% luminal narrowing and only 8.5% of cases had less than 50% involvement in LCX. More than 70% involvement in RCA was seen in 74.6% of cases and 13.4% of cases had less than 50% involvement.
All 4% of the patients with the involvement of the left main artery had type C lesions. Type C lesion was the most common finding in all the vessels which is 54.2%, 58.9%, and 68.7% in LAD, LCX, and RCA respectively. This was similar to the study by Avinash Mani showed the majority of participants had multivessel disease on angiography with LAD being the most common culprit vessel. 45% of patients had complex lesion morphology (ACC/AHA Type B/C) on angiography [43].
According to Huang 2019, et al. the optimal cut-off value of MPV in predicting ACS was 10.55 Fl (sensitivity: 54.2%; specificity: 83.8%) [28]. The Positive Predictive Value (PPV) and negative predictive value (NPV) of MPV in diagnosing ACS was 84.2% and 53.4%, respectively, which is almost comparable to our study with the cutoff of MPV of 10.6 fl with a sensitivity of 84% and specificity of 50%. The positive Predictive Value (PPV) of 89% and NPV of 38% with a diagnostic accuracy of 79% and IPF cut of 2.9 had a sensitivity of 90.5% with a specificity of 50%, positive predictive value (PPV) of 90.5% and negative predictive value (NPV) of 50% with a diagnostic accuracy of 84% for predicting involvement of left anterior descending artery. Sansanayudh, et al. showed that patients with high MPV have 2.28 times significantly higher odds of having CAD than patients with low MPV [44]. The AUROC for IPF (%) predicting involvement of LAD was 0.738 (95% CI: 0.595 - 0.882), thus demonstrating fair diagnostic performance which was statistically significant (p = 0.003), and the AUROC for MPV (fL) in predicting involvement of LAD was 0.672 (95% CI: 0.515 - 0.83) which was statistically significant (p = 0.030). A cutoff of MPV (fL) ≥10.6 can predict the involvement of LAD with a sensitivity of 84%, and a specificity of 50%. With a positive predictive value of 38.1% and a negative predictive value of 38.1% however, in our study area under the curve analysis for IPF and MPV for LCX, RCA, left main were not statistically significant and did not show fair diagnostic performance.
In our study mean value of MPV of 10.04 ± 0.89 in TVD was greater than the mean value of MPV of 9.22 ± 0.67 in DVD and that was statistically significant (p < 0.05) The AUROC for MPV (fL) predicting TVD was 0.629 (95% CI: 0.515 - 0.742) which was statistically significant (p = 0.030). At a cutoff of MPV (fL) ≥ 14.2 predicts TVD with a sensitivity of 30%, and a specificity of 93%, thus MPV (fL) significantly predicts TVD. The best parameter for predicting TVD in terms of AUROC, sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy was MPV (fL). Similar to the results by Pratia [16] who also stated that IPF may be a more sensitive and specific diagnostic marker than MPV to evaluate platelet turnover in ACS patients [5]. Mean IPF values and MPV levels were significantly higher in patients with LAD involvement i.e., 4.40 ± 1.72% and 12.45 ± 1.88 respectively than in patients without involvement of LAD i.e., 2.78 ± 1.50% (p = 0.003) and 11.08 ± 2.19 (p = 0.030) respectively). Some factors associated with LAD lesions such as lower LVEF and higher inflammation due to extensive necrosis might be triggers of higher MPV in NSTEMI with LAD culprit lesions. These results suggest that MPV and IPF may be used as early independent markers for the extent of CAD in NSTEMI patients. Similar to our findings in a study conducted by Liu, et al. by studying 190 patients with NSTEMI they found that MPV was a reliable efficient tool in predicting LAD obstruction in NSTEMI patients [45].
Limitations
The first limitation is the use of an automated and standardized instrument to detect MPV and IPF, which could provide test results with faster diagnosis, better precision, and reproducibility. However, the normal range of MPV may be variable in different instruments or races, so each lab should establish its cut-off value. Secondly, to properly measure MPV and IPF, the timing of the test must be strictly controlled because the MPV increases with anticoagulant placement time and decreases due to the dilution of cytoplasmic content. Third, some reports indicate that MPV and IPF are related to cardiovascular mortality. However, since the sample size in this study was limited and lack of follow-up, we did not observe the correlation between mortality and these biomarkers. Therefore, the relevance of these biomarkers to patient outcomes may be further addressed by a large sample size, following up, and conducting multi-center studies.
According to the high sensitivity of Immature Platelet Fraction (IPF) and Mean Platelet Volume (MPV) and as simple inexpensive tests, they could be suggested as a valuable screening test in Non-ST segment Elevation Myocardial Infarction (NSTEMI) patients in predicting the involvement of Left Anterior Descending Artery (LAD). Patients with NSTEMI with high MPV had significantly high IPF, and lower platelet count, was significantly associated with involvement in LAD, and had a positive correlation with Triple Vessel Disease. Prospective, large-scale studies should identify the usefulness of adding IPF and MPV to current risk stratification scores in patients with NSTEMI. Our study suggests that MPV and IPF may be useful early independent hematologic markers to easily identify patients with a higher risk for significant CAD in patients with NSTEMI. These results help to distinguish which patients are at high risk of significant CAD, and so more likely to benefit from invasive studies from those with low risk who may need further noninvasive workups.
- Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol. 2013 Sep 30;168(2):934-45. doi: 10.1016/j.ijcard.2012.10.046. Epub 2012 Dec 4. PMID: 23218570; PMCID: PMC3819990.
- Jameson J, Fauci AS, Kasper DL. Harrison’s Principle of Internal Medicine. 1867; 2.
- Jneid H. The 2012 ACCF/AHA Focused Update of the Unstable Angina/Non-ST-Elevation Myocardial Infarction (UA/NSTEMI) Guideline: a critical appraisal. Methodist Debakey Cardiovasc J. 2012 Jul-Sep;8(3):26-30. doi: 10.14797/mdcj-8-3-26. PMID: 23227283; PMCID: PMC3487574.
- Diver DJ, Bier JD, Ferreira PE, Sharaf BL, McCabe C, Thompson B, Chaitman B, Williams DO, Braunwald E. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial). Am J Cardiol. 1994 Sep 15;74(6):531-7. doi: 10.1016/0002-9149(94)90739-0. PMID: 8074033.
- van der Loo B, Martin JF. A role for changes in platelet production in the cause of acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1999 Mar;19(3):672-9. doi: 10.1161/01.atv.19.3.672. PMID: 10073972.
- Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, Wilczynska J, Zielinski A, Meier B, Opolski G. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005 Jul 19;46(2):284-90. doi: 10.1016/j.jacc.2005.03.065. PMID: 16022956.
- Norgaz T, Hobikoglu G, Aksu H, Bolca O, Uyarel H, Eren M, Narin A. The relationship between preprocedural platelet size and subsequent in-stent restenosis. Acta Cardiol. 2004 Aug;59(4):391-5. doi: 10.2143/AC.59.4.2005204. PMID: 15368800.
- Turakhia MP, Murphy SA, Pinto TL, Antman EM, Giugliano RP, Cannon CP, Braunwald E, Gibson CM; Thrombolysis in Myocardial Infarction Study Group. Association of platelet count with residual thrombus in the myocardial infarct-related coronary artery among patients treated with fibrinolytic therapy for ST-segment elevation acute myocardial infarction. Am J Cardiol. 2004 Dec 1;94(11):1406-10. doi: 10.1016/j.amjcard.2004.08.015. PMID: 15566912.
- Jose R, Endler G, Lalouscheck W. Mean platelet volume; A potential biomarker ofthe risk and prognosis of heart disease. Journal of Polandia. 2016; 31:1009-17. 10.3904/kjim.2016.078
- Hoffmann JJ. Reticulated platelets: analytical aspects and clinical utility. Clin Chem Lab Med. 2014 Aug;52(8):1107-17. doi: 10.1515/cclm-2014-0165. PMID: 24807169.
- Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, DeLao T, Granada JF, Dong JF, Kleiman NS, Lev EI. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol. 2008 Aug 26;52(9):743-9. doi: 10.1016/j.jacc.2008.05.031. PMID: 18718422.
- Perl L, Lerman-Shivek H, Rechavia E, Vaduganathan M, Leshem-Lev D, Zemer-Wassercug N, Dadush O, Codner P, Bental T, Battler A, Kornowski R, Lev EI. Response to prasugrel and levels of circulating reticulated platelets in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014 Feb 18;63(6):513-7. doi: 10.1016/j.jacc.2013.07.110. Epub 2013 Oct 30. PMID: 24148715.
- De Luca G, Verdoia M, Cassetti E, Schaffer A, Cavallino C, Bolzani V, Marino P; Novara Atherosclerosis Study Group (NAS). High fibrinogen level is an independent predictor of presence and extent of coronary artery disease among Italian population. J Thromb Thrombolysis. 2011 May;31(4):458-63. doi: 10.1007/s11239-010-0531-z. PMID: 21080031.
- Pons I, Monteagudo M, Lucchetti G, Muñoz L, Perea G, Colomina I, Guiu J, Obiols J. Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. Eur J Haematol. 2010 Aug;85(2):158-63. doi: 10.1111/j.1600-0609.2010.01468.x. Epub 2010 May 8. PMID: 20477866.
- Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, Widimsky P, McCullough PA, Hunt D, Braunwald E, Yusuf S. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. 2005 Jun 15;293(23):2908-17. doi: 10.1001/jama.293.23.2908. PMID: 15956636.
- Paramita P, Nurulita A, Pakasi RD. Analysis of immature platelet fraction and mean platelet volume in acute coronary syndrome patient. Young. 2019; 1:2.
- Sari EB, Akbar NZ, Hariman H. Immature Platelet Fraction (IPF) Levels in Acute Coronary Syndrome (ACS) Patients. IJRR. 2015; 8(8):682-8.
- Khan R, Akhter J, Munir U, Almas T, Ullah W. Frequency of Non-ST Segment Elevation Myocardial Infarction (NSTEMI) in Acute Coronary Syndrome With Normal Electrocardiogram (ECG): Insights From a Cardiology Hospital in Pakistan. Cureus. 2020 Jun 22;12(6):e8758. doi: 10.7759/cureus.8758. PMID: 32714696; PMCID: PMC7377671.
- Ibrahim H, Schutt RC, Hannawi B, DeLao T, Barker CM, Kleiman NS. Association of immature platelets with adverse cardiovascular outcomes. J Am Coll Cardiol. 2014 Nov 18-25;64(20):2122-9. doi: 10.1016/j.jacc.2014.06.1210. Epub 2014 Nov 10. PMID: 25457402.
- Stratz C, Bömicke T, Younas I, Kittel A, Amann M, Valina CM, Nührenberg T, Trenk D, Neumann FJ, Hochholzer W. Comparison of Immature Platelet Count to Established Predictors of Platelet Reactivity During Thienopyridine Therapy. J Am Coll Cardiol. 2016 Jul 19;68(3):286-293. doi: 10.1016/j.jacc.2016.04.056. PMID: 27417007.
- Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010 Jan;8(1):148-56. doi: 10.1111/j.1538-7836.2009.03584.x. Epub 2009 Aug 19. PMID: 19691485; PMCID: PMC3755496.
- Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, Wilczynska J, Zielinski A, Meier B, Opolski G. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005 Jul 19;46(2):284-90. doi: 10.1016/j.jacc.2005.03.065. PMID: 16022956.
- Pabón Osuna P, Nieto Ballesteros F, Moríñigo Muñoz JL, Sánchez Fernández PL, Arribas Jiménez A, Diego Domínguez M, Martín Luengo C. Influencia del volumen plaquetario medio sobre el pronóstico a corto plazo del infarto agudo de miocardio [The effect of the mean platelet volume on the short-term prognosis of acute myocardial infarct]. Rev Esp Cardiol. 1998 Oct;51(10):816-22. Spanish. PMID: 9834631.
- Ghaffari S, Pourafkari L, Javadzadegan H, Masoumi N, Jafarabadi MA, Nader ND. Mean platelet volume is a predictor of ST resolution following thrombolysis in acute ST elevation myocardial infarction. Thromb Res. 2015 Jul;136(1):101-6. doi: 10.1016/j.thromres.2015.05.003. Epub 2015 May 10. PMID: 25987395.
- Kırbaş Ö, Kurmuş Ö, Köseoğlu C, Duran Karaduman B, Saatçi Yaşar A, Alemdar R, Ali S, Bilge M. Association between admission mean platelet volume and ST segment resolution after thrombolytic therapy for acute myocardial infarction. Anadolu Kardiyol Derg. 2014 Dec;14(8):728-32. doi: 10.5152/akd.2014.5078. Epub 2014 Apr 2. PMID: 25188762.
- Grove EL, Hvas AM, Kristensen SD. Immature platelets in patients with acute coronary syndromes. Thromb Haemost. 2009 Jan;101(1):151-6. PMID: 19132202.
- Berny-Lang MA, Darling CE, Frelinger AL 3rd, Barnard MR, Smith CS, Michelson AD. Do immature platelet levels in chest pain patients presenting to the emergency department aid in the diagnosis of acute coronary syndrome? Int J Lab Hematol. 2015 Feb;37(1):112-9. doi: 10.1111/ijlh.12250. Epub 2014 May 8. PMID: 24806286; PMCID: PMC4225001.
- Huang HL, Chen CH, Kung CT, Li YC, Sung PH, You HL, Lin YH, Huang WT. Clinical utility of mean platelet volume and immature platelet fraction in acute coronary syndrome. Biomed J. 2019 Apr;42(2):107-115. doi: 10.1016/j.bj.2018.12.005. Epub 2019 May 8. PMID: 31130246; PMCID: PMC6541877.
- Ahamed H, Henry RA, Pai R. Association of mean platelet volume and acute coronary syndrome. Int. J Res Med Sci. 2017; 5(4):1217-20.
- Nardin M, Verdoia M, Negro F, Rolla R, Tonon F, De Luca G. Impact of active smoking on the immature platelet fraction and its relationship with the extent of coronary artery disease. Eur J Clin Invest. 2020 Feb;50(2):e13181. doi: 10.1111/eci.13181. Epub 2020 Jan 18. PMID: 31659742.
- Taskesen T, Sekhon H, Wroblewski I, Goldfarb M, Ahmad MB, Nguyen QT, Fughhi IA, Gidron A, Dadkhah S. Usefulness of Mean Platelet Volume to Predict Significant Coronary Artery Disease in Patients With Non-ST-Elevation Acute Coronary Syndromes. Am J Cardiol. 2017 Jan 15;119(2):192-196. doi: 10.1016/j.amjcard.2016.09.042. Epub 2016 Oct 8. PMID: 27814786.
- Hardy M, Lessire S, Kasikci S, Baudar J, Guldenpfennig M, Collard A, Dogné JM, Chatelain B, Jacqmin H, Lecompte T, Mullier F. Effects of Time-Interval since Blood Draw and of Anticoagulation on Platelet Testing (Count, Indices and Impedance Aggregometry): A Systematic Study with Blood from Healthy Volunteers. J Clin Med. 2020 Aug 4;9(8):2515. doi: 10.3390/jcm9082515. PMID: 32759828; PMCID: PMC7465339.
- Gang L, Yanyan Z, Zhongwei Z, Juan D. Association between mean platelet volume and hypertension incidence. Hypertens Res. 2017 Aug;40(8):779-784. doi: 10.1038/hr.2017.30. Epub 2017 Mar 9. PMID: 28275234.
- Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005 Aug;59(8):981-2. doi: 10.1111/j.1742-1241.2005.00500.x. PMID: 16033624.
- Kario K, Matsuo T, Nakao K. Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol. 1992;14(4):281-7. doi: 10.1111/j.1365-2257.1992.tb00103.x. PMID: 1478007.
- Nadar S, Blann AD, Lip GY. Platelet morphology and plasma indices of platelet activation in essential hypertension: effects of amlodipine-based antihypertensive therapy. Ann Med. 2004;36(7):552-7. doi: 10.1080/07853890410017386. PMID: 15513305.
- Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, Lakasas G. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004 Dec;15(8):475-8. doi: 10.1080/0953710042000267707. PMID: 15763888.
- Pathansali R, Smith N, Bath P. Altered megakaryocyte-platelet haemostatic axis in hypercholesterolaemia. Platelets. 2001 Aug;12(5):292-7. doi: 10.1080/09537100120058810. PMID: 11487381.
- Yazici HU, Poyraz F, Sen N, Tavil Y, Turfan M, Tulmaç M, Abacı A. Relationship between mean platelet volume and left ventricular systolic function in patients with metabolic syndrome and ST-elevation myocardial infarction. Clin Invest Med. 2011 Dec 1;34(6):E330. doi: 10.25011/cim.v34i6.15892. PMID: 22129921.
- Şenel E, Acar B, Demir E. Mean Platelet Volume: A Reliable Marker of Inflammation in Recurrent Apthous Stomatitis and Behçet Disease? Indian Dermatol Online J. 2017 Nov-Dec;8(6):468-470. doi: 10.4103/idoj.IDOJ_405_16. PMID: 29204391; PMCID: PMC5707840.
- Desai A, Bhagarhatta R. Localization of Angina Related Artery by Admission ECG in Unstable Angina and NSTEMI Patients. J Hypertens Cardiol. 2016; 2:10-6. 10.14302/issn.2329-9487.jhc-13-335
- Separham A, Shahsavani A, Sarvestani AH: Study of the Relationship between Mean Platelet Volume and. LAD as a Culprit Vessel in NSTEMI. Multidisciplinary Cardiovascular Annals. 2020; 11: 31. 10.5812/mca.100194
- Mani A: Optical coherence tomography based characterisation of culprit lesions in acute coronary syndrome. Diss SCTIMST. 2020:25-66.
- Sansanayudh N, Anothaisintawee T, Muntham D, McEvoy M, Attia J, Thakkinstian A. Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2014 Aug 20;175(3):433-40. doi: 10.1016/j.ijcard.2014.06.028. Epub 2014 Jun 28. Erratum in: Int J Cardiol. 2014 Dec 20;177(3):1145. AmmarinThakkinstian [corrected to Thakkinstian, Ammarin]. PMID: 25017904.
- Liu Q, Wang T, Chen R, Liu C, Yue W, Hong J, Jia R. Mean platelet volume predicts left descending artery occlusion in patients with non-ST-elevation myocardial infarction. Platelets. 2014;25(4):246-51. doi: 10.3109/09537104.2013.810332. Epub 2013 Oct 8. PMID: 24102229.