More Information
Submitted: October 11, 2023 | Approved: October 18, 2023 | Published: October 19, 2023
How to cite this article: Kumar V. Preventing Coronary Occlusion in an Elderly Severe Aortic Stenosis Patient with Critically Low Coronary Heights – A Case Report. J Cardiol Cardiovasc Med. 2023; 8: 130-136.
DOI: 10.29328/journal.jccm.1001165
Copyright License: © 2023 Kumar V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Annulus rupture; Balloon expandable; Coronary obstructions; Low coronary height; Myval; Small annulus; Transcatheter heart valve
Preventing Coronary Occlusion in an Elderly Severe Aortic Stenosis Patient with Critically Low Coronary Heights – A Case Report
Viveka Kumar*
Max Super Speciality Hospital, Saket, New Delhi, India
*Address for Correspondence: Dr. Viveka Kumar, MD, General Medicine, DM-Cardiology, Max Super Speciality Hospital, Saket, New Delhi, 110017, India, Email: [email protected]
Background: Transcatheter Aortic Valve Replacement (TAVR) is an established treatment for severe, symptomatic Aortic Stenosis (AS). However, the presence of low coronary heights confers a high risk for coronary obstruction during or after TAVR.
Case: In this case report, we present our experience with transfemoral-TAVR in an elderly, high-risk (STS score – 12.08%) female severe AS patient with low coronary heights (right: 7.4 mm, left: 8.7 mm). She had lower annulus area (287 mm2) and moderately low valve area (0.7 cm2) as well. Her mean and peak pressure gradients (PGs) were 38 mmHg and 61 mmHg, respectively. Upon the Heart Team’s evaluation, TAVR was recommended and a 20 mm Balloon Expandable (BE) Myval Transcatheter Heart Valve (THV) was selected. No peri-procedural or post-procedural complications were reported and the post-procedural hemodynamics, namely the mean and peak PGs improved to 16 mmHg and 30 mmHg after TAVR, respectively. The patient was discharged in a stable condition after four days of hospital stay.
Conclusion: We report the successful implantation of a small-sized BE Myval THV (20 mm) in a patient with low coronary heights. Life-threatening complications including paravalvular leak, coronary obstruction, or annular rupture were well averted; hence, we ascertain that the Myval THV is a suitable device for treating severe AS in difficult anatomies. However, the viability of the novel valve needs to be reaffirmed in larger studies..
Among all valvular heart diseases, degenerative calcific Aortic Stenosis (AS) and mitral valve disease affect 9 and 24 million people, respectively [1]. Asia has the highest prevalence of rheumatic Aortic Stenosis (AS), with prevalence of 4.54, 1.86, and 1.3 per 1000 people in India, China, and Bangladesh, respectively. Because of the rising prevalence of AS along with the effects of population aging, the clinical attention on VHD and particularly on AS has increased considerably. While there are more VHD cases among women than among men globally, it has been observed that large trials included more men [2]. According to the Global Burden of Valvular Heart Disease Report, the majority of patients (81.9%) with AS have a degenerative-calcific etiology, while 11.2% have rheumatic etiology, 5.6% have congenital etiology, and 1.3% have AS post endocarditis [3].
Earlier, Surgical Aortic Valve Replacement (SAVR) was the benchmark of AS management. However, elderly patients having multiple comorbidities are generally unsuitable for SAVR because of the high predicted risk of 30-day mortality [4]. The development of transcatheter devices and the progress in clinical experience of Transcatheter Aortic Valve Replacement (TAVR), coupled with the accumulation of high-valued evidence on TAVR have altogether given a strong impetus to interventional cardiology. With this transformation in the clinical practice management scenario, TAVR has gained widespread adoption and prominence in the routine clinical management of severe, symptomatic AS, especially for patients aged above 65 years [5]. The first case of TAVR was performed in the year 2002 by Alan Cribier. Since then, the Transcatheter Heart Valves (THVs) have undergone massive evolution in terms of device design. Moreover, the landmark PARTNER trials have exhibited TAVR to be a feasible treatment option for low, intermediate, and high-risk AS patients [6].
When the coronary height is below 10 mm, it is considered an anatomical predictor of coronary occlusion during TAVR [7]. Recent research suggests that patients with low coronary heights (≤ 7 mm) can safely undergo TAVR using Self-Expandable (SE) THV with a high rate of procedural success and no acute coronary obstructions [7].
Myval™ Transcatheter Heart Valve (THV) (Meril Life Sciences Pvt. Ltd., Gujarat, India) is a Conformité Européenne (CE) certified, balloon-expandable (BE), newer-generation valve with tri-leaflet bovine pericardial leaflets. The THV is crimped over a high-flexion Navigator THV delivery system (Meril Life Sciences Pvt. Ltd., India) [8]. The efficacy and safety of BE Myval THV have been demonstrated in different studies such as in severe bicuspid AS patients, where short-term favorable hemodynamic and clinical results were observed [9] and in the TRITON-TAVI registry, Amat-Santos, et al. showed that Myval had a relatively improved gradient in comparison to Evolut Pro+ and SAPIEN 3 Ultra [10]. In comparison to Evolut SE, this BE valve has exhibited comparable clinical performance a decreased risk of Paravalvular Leakage (PVL), and a lower need for Permanent Pacemaker Implantation (PPI) within 30 days and 6 months post-TAVR [11] Moreover, this THV demonstrated a favorable hemodynamic performance in low-risk AS patients [12]. Furthermore, according to the quantitative angiographic results of a retrospective Core Lab analysis, the BE Myval THV has shown less severe or moderate aortic regurgitation than other BE valves [13]. As per a blinded electrocardiographic analysis, BE Myval THV was associated with low rates of conduction disturbances as compared to other devices [14]. In this report, we investigate the possibility of TAVR using BE Myval THV on a high-risk female patient who had severe AS and low coronary heights.
An 81-year-old female patient was admitted to our center due to retrosternal chest pain that tended to increase on exertion. The patient had hypertension and was non-diabetic, and normotensive. Her cardiac history revealed the implantation of a permanent pacemaker. The vital signs such as pulse rate were found to be 80 beats per minute, blood pressure - 140/80 mmHg, and SpO2 - 88% on room air. Pre-procedural transthoracic echo-doppler analysis showed a mean pressure gradient (PG) of 38 mmHg, peak PG of 61 mmHg, aortic valve area of 0.7 cm2, which indicates severe AS, and Left Ventricular Ejection Fraction (LVEF) of 55%. The patient had severe regurgitation of the tricuspid valve, which can be correlated to the detection of Right Ventricular Systolic Pressure (RVSP) of 50 mmHg and Pulmonary Arterial Systolic Pressure (PASP) of 35 mmHg. The patient also had mild regurgitation spotted in the mitral valve. As per the Multi-Slice Computed Tomography (MSCT) report generated using the 3mensio Structural Heart software (Pie Medical Imaging BV, AJ Maastricht, The Netherlands), the diameters of the aortic annulus, aortic root, and ascending aorta were 1.9, 2.8 and 2.3 cm, respectively. A dilated left atrium (4.5 cm) and normal-sized left ventricle were observed. However, grade II diastolic dysfunction was noted. The aorta at the sinus of Valsalva was smaller (2.6 cm) than the normal range for women (3 cm - 3.6 cm), while the left atrium was slightly larger (4.6 cm) than the normal range for women (2.7 cm - 3.8 cm).
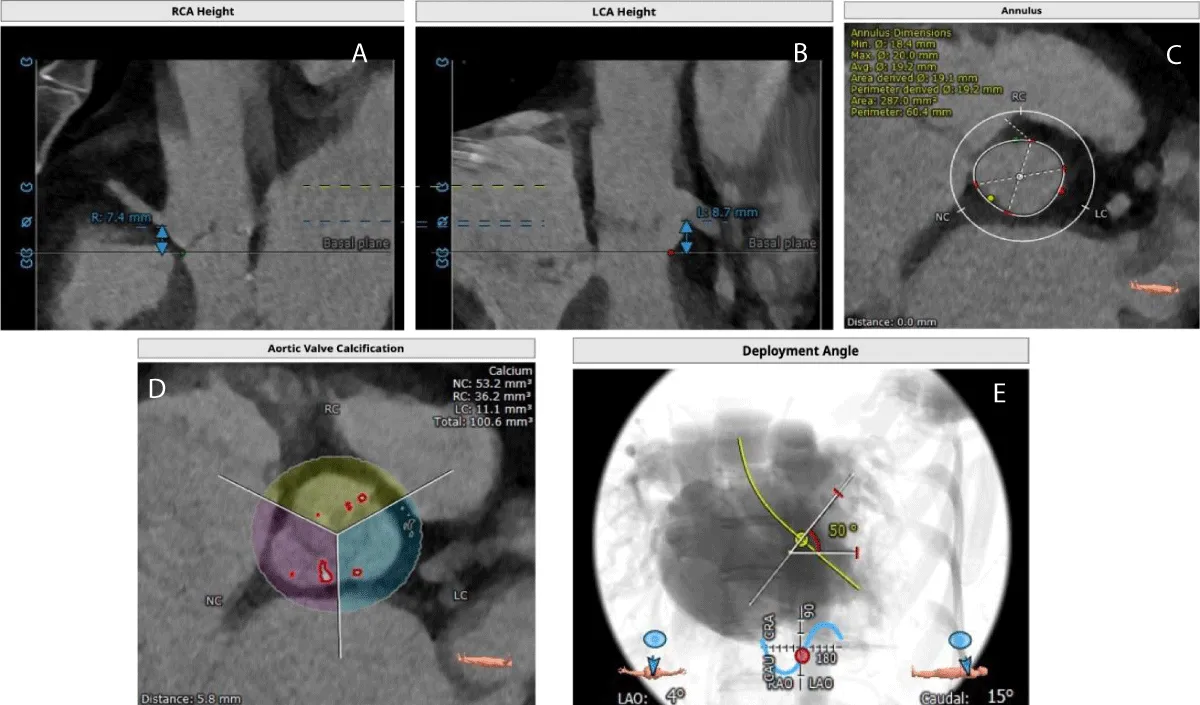
The MSCT scan showed Coronary Arteries (CA) with low heights (right CA: 7.4 and left CA: 8.7 mm) (Figure 1). The patient was considered suitable for transfemoral TAVR as per the Heart Team’s decision and a 20 mm BE Myval THV was selected in consideration of the low coronary heights. Written informed consent was obtained from the patient and the family.
Figure 1: Computed Tomography images shows- A) Right coronary artery height, B) Left coronary artery height, C) Aortic annulus area, D) Aortic valve calcification and E) Deployment angles.
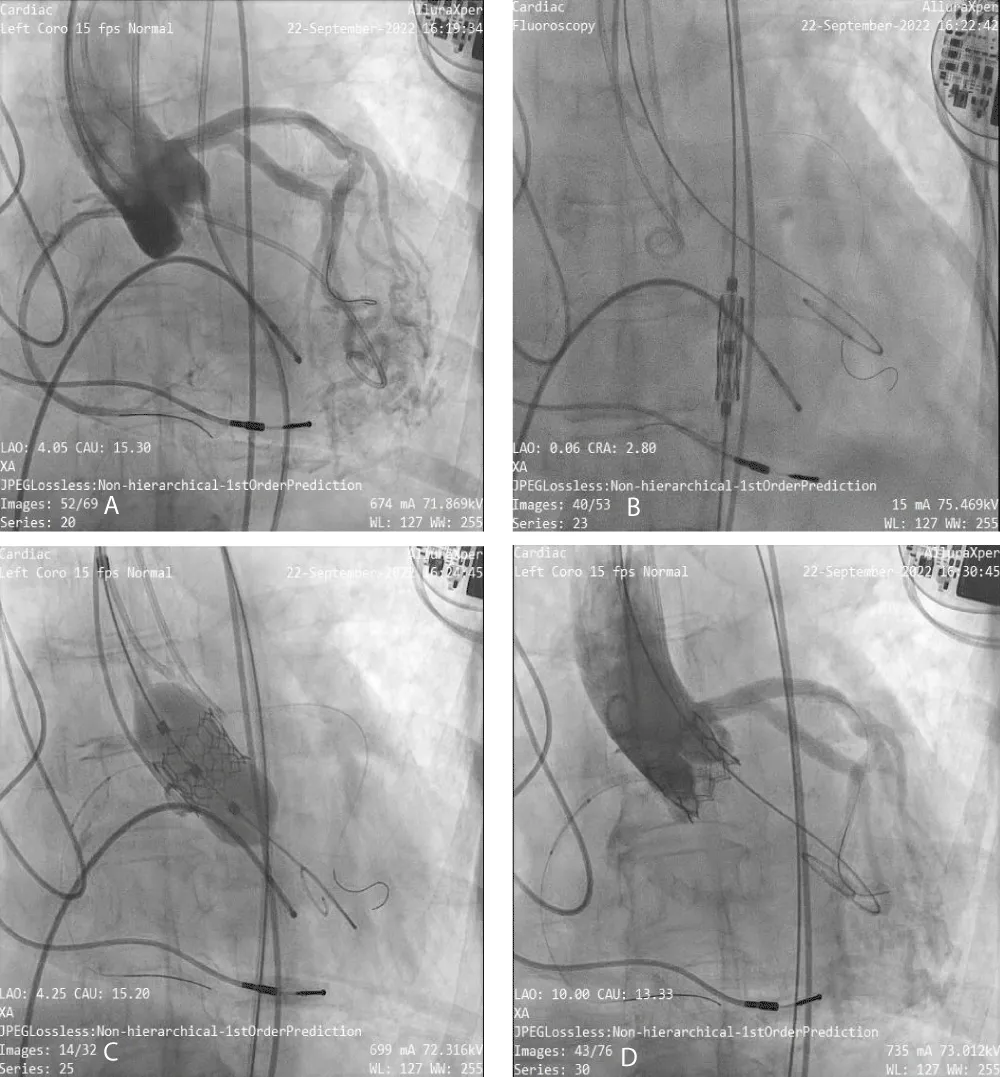
The TAVR was performed in the patient under conscious sedation and angiographic imaging guidance with transthoracic echo-doppler monitoring. The right and left femoral arteries, and right femoral vein were cannulated. Through the right femoral vein access, the Temporary Pacemaker (TPI) was positioned in the right ventricle. As a marker for valve installation and to permit arteriography for positioning during the TAVR treatment, a 6-Fr graded pigtail catheter was inserted into the noncoronary sinus via the right femoral artery. Following the completion of all arterial and venous accesses, intravenous unfractionated heparin was delivered to achieve the necessary activated clotting time of > 250 s. The right femoral artery was used to insert a soft 0.035”- J-tipped wire into the descending thoracic aorta followed by performing a pre-closure using a single suture-mediated closure device (Perclose ProGlide®, Abbott Laboratories, Abbott Park, IL, USA) while maintaining arterial access through the J-tipped guidewire. Serial dilatations were performed using the 10-Fr/12-Fr dilator, which was followed by the insertion of the 14-Fr Python Introducer Sheath into the right femoral artery. To cross the valve, a 6-Fr AL-1 catheter was inserted through the valve delivery sheath, and a 0.035”- J-tipped guidewire was inserted and swapped for a 0.035 - inch straight-tip wire (Radiofocus® Guidewire M, Terumo Corporation., Tokyo, Japan). After crossing, the straight-tip wire was replaced with a 300-cm J-tipped wire following which, the AL-1 catheter was withdrawn and replaced with a 6-Fr angled pigtail catheter. Subsequently, a stiff guidewire SAFARI 2™ (Boston Scientific, Marlborough, MA, USA) was inserted through the angled pigtail catheter into the left ventricle with the guidewire’s transition point held above the apex and pointing away from the ventricular wall. A 20 mm BE Myval THV implant was crimped over the Navigator THV delivery system and deployed with rapid pacing through the TPI (Figure 2). Immediately after the procedure, peak and mean PG improved to 30 mmHg (baseline: 61 mmHg) and 16 mmHg (baseline: 38 mmHg), respectively.
Figure 2: Angiographic images of Myval THV Implantation- A) Baseline Aortogram, B) Myval THV Navigation, C) Myval Deployment and D) Final aortogram with no PVL.
In-hospital outcomes
After the procedure, the patient displayed stable hemo-dynamic parameters and exhibited no major complications. Post implantation, the operator confirmed a good valve position and coronary perfusion without any evidence of aortic regurgitation or coronary occlusion. Hence, the Navigator delivery system, TPI, and all guidewires were removed with the activated clotting time < 170 s. Right and left femoral access sites were closed using 2 Perclose ProGlide® sutures (Abbott Laboratories, Abbott Park, Illinois, USA) and one 8 Fr Angio-Seal (St Jude Medical, St Paul, Minnesota, USA).
At discharge, the following medications were prescribed: Clopidogrel 75 mg, Ecosprin 75 mg once daily (at night-time), Neksium 40 mg once daily, and Intravenous Potassium Chloride infusion and Magnex 1 gm. After completing the hospital stay for four days without exhibiting any complications such as life-threatening bleeding, paravalvular leak, or significant vascular complication, the patient was discharged in stable condition.
Device description
The Myval THV is a stented THV having aCobalt–Chromium alloy frame, incorporating three AntiCa®-treated bovine pericardial tissue leaflets and internal and external Polyethylene Terephthalate (PET) fabric that offers better puncture resistance and minimizes paravalvular leakage. The THV incorporates a hybrid honeycomb design composed of upper open cells (53%) and lower closed cells (47%). In particular, the upper open cells ensure the un-jailing of coronary ostia with the capacity to accommodate the highest cusp annulus diameter of 6 mm, while the closed cells in the lower portion provide radial strength. The broad size matrix of Myval THV comprises conventional (20, 23, 26, and 29 mm), intermediate (21.5, 24.5, and 27.5 mm), and extra-large sizes (30.5 and 32 mm), which facilitates the optimal sizing of the THV for avoiding annular rupture or unnecessary over-dilatation or THV migration during deployment, and coronary obstruction [8,15]. Prior to insertion, the valve was mounted and crimped on the over-the-wire balloon catheter of the high-flexion Navigator delivery system provided in the Myval accessory kit. When crimped, the open and closed cells create a distinctive alternate dark-light band-like pattern that enables precise THV deployment throughout the original annulus setting [16]. The Myval THV has a short frame height (17 mm - 21 mm) [8] as compared to other contemporary devices [17] which makes it suitable for patients with low coronary heights and prevents coronary obstruction. The frame height of 20 mm Myval is 17.35 mm, which covers a three-dimensional annular area of 287 mm2.
Symptomatic AS can be fatal, if not treated at the appropriate time [18]. As per the recent clinical practice guidelines of the American College of Cardiology/American Heart Association, TAVR is recommended for treating severe symptomatic AS in all adults, regardless of the surgical risk [19]. Our report presents the case of a high-risk 81-year-old female patient with severe AS, small annulus area, and low heights of both left and right coronary ostia who underwent TAVI with successful post-procedural outcomes using BE Myval THV (size 20 mm).
Low coronary height is a risk factor for coronary obstruction, which is a fatal complication of TAVR. Although rare (< 4%) in the current times, acute coronary obstruction requires immediate peri-operative attention. The possible risk factors for coronary obstruction and their appropriate solutions are presented in Table 1. In our patient, the annulus was noted to be smaller than the normal sizes, as can be expected in South Asian populations. This is a notable factor since a smaller annulus is independently associated with the risk of prosthesis-patient mismatch [20]. Another factor that needs imperative consideration is the presence of a smaller body size, which has a potential risk for annulus rupture [20].
| Table 1: Complications during TAVR and characteristics of Myval THV. | ||||
| Risk factor | Complication | Probable Solution | Myval THV Characteristics | Reference |
|
Coronary obstruction following TAVR (due to displacement of the calcified native cusp over the coronary ostia) | Retrievable valve, low frame height | It is fully retrievable when undeployed and is available in a variety of sizes with a low frame height of about 17 mm - 21 mm | [8,27] |
| 1. Smaller annular area (< 300 cm2) | Annular rupture due to relative valve oversizing | Valve with small size | It is available in 20 mm size which is suitable for < 300 cm2 | [8,29] |
| 1. Patient - prosthesis size mismatch (due to small annuus area or low coronary height) | Paravalvular leakage | Valve with appropriate size as the annulus | It is for small annulus size with a low frame height suitable for low coronary height (20 mm size suitable for < 300 cm2 with 17.35 mm frame height) | [8,20] |
| TAVR: Transcatheter Aortic Valve Replacement; VTC: Valve-To-Coronary distance measured on virtual reconstructive MS-CT analysis. | ||||
In the Karlsruhe registry, Conzelmann, et al. compared ACCURATE Neo, CoreValve, Lotus, Portico, and SAPIEN XT/S3 THVs in patients having calcified AS in patients with an average distance of 6.4 ± 1.1 mm between the coronary and annulus planes. Coronary obstruction was reported in 3.5% of patients while 1.1% of cases of intraoperative death occurred [21]. The study also showed that in 24.4% of patients who received a BE THV, no coronary obstruction had occurred. In particular, this registry reported that 68.6% (n = 59) of the THV recipients were females [21]. In a meta-analysis by Akinseye, et al. on the outcomes of patients undergoing TAVR, the prevalence of implanting BE THVs was 78%. Yamamoto, et al. investigated the outcomes of performing pre-emptive coronary protection in patients having high-risk aortic root anatomical characteristics indicated for TAVR. In this study, 70.4% of patients were women, and a short stature and low average BMI (22.2 kg/m2) was observed. Furthermore, among the 1.5% incidence of acute coronary obstruction reported (n = 10), 70% were women [22]. In the meta-analysis by Akinseye, et al. 40 studies reporting 96 cases of coronary occlusion associated with TAVR were included, and the authors remarked that 81% of such patients were women of advanced age (> 70 years) [23]. In an earlier clinical study of a total of 269 participants with a mean coronary height of 8.9 ± 1.2 mm who underwent TAVR, low coronary heights were reported among 10.8% of patients, of whom 24 patients (82.8%) were women [7]. Hence, although not significantly pointed out in the literature, the female gender seems as a considerable factor predisposed to coronary occlusion during TAVR, and therefore, the choice of THV selection becomes inevitably important to ensure successful TAVR outcomes. Moreover, this factor assumes greater clinical importance in Asian populations, in whom the short stature and low heights of sinus of Valsalva, sino-tubular junction, and lower coronary artery ostial heights are prevalent [24-26].
The relationship between cusp height and coronary height is the primary metric for assessing the risk of coronary obstruction. Consequently, if the lowest plane of the coronary artery is lower than the commissural attachment of the aortic cusp, or if the Virtual THV-To-Coronary (VTC) distance is ≤ 4 mm, or calcium volume > 600 mm3 in the culprit leaflet, the risk of coronary obstruction increases [20-27].
In this case experience, successful implantation of BE Myval THV has been demonstrated in high-risk patients with low coronary heights and a small annulus, in whom the THV selection was a critical consideration. Myval THV is a newer-generation, BE device that received approval from the Drug Controller General of India (DCGI) in October 2018 and was CE-marked in 2019 [8]. In a blinded echocardiographic study involving 461 real-world patients with severe AS, Myval THV, when compared to SAPIEN 3 (Edwards Lifesciences, USA), was associated with a higher procedural success rate (93.2% vs. 94.2% for Myval vs. SAPIEN 3, p = 0.219), better early safety (4.9% vs. 12.6%, p = 0.096), lower 30-day mortality (0.97% vs. 2.9%, p = 0.625), improved clinical efficacy (4.9% vs. 12.6%,
p = 0.057), and lower rate of permanent pacemaker placement (5.8% vs. 15.5%, p = 0.020) [28]. Another study evaluated the performance of Myval THV in 100 low-risk AS patients and the data showed good hemodynamic performance and favorable results in 30-day clinical outcomes without any severe mismatch cases, less residual aortic regurgitation, and lower rates of moderate PVL [12]. In the current case, accurate positioning and deployment were possible because of the THV’s light and dense banding patterns that ensured the precise landing zone. The risks of conduction system disturbances are minimized because of the short infra-annular depth on the aortic part of the valve (≤ 3.5 mm), which helps reduce the need for permanent pacemaker dependency. The low-profile design of the Myval THV enables optimal anchorage with minimal LVOT footprint without the risk of THV migration. Furthermore, the selection of 20 mm THV size was quite appropriate for this patient, given that she had a small annulus area and a smaller body size, along with low coronary heights on the left and right sides. The 20 mm Myval THV has a very low frame height (17.35 mm), which enables its low profile deployment with the least chances of THV migration, thus enabling maximum protection against annulus rupture, acute coronary occlusion, prosthesis-patient mismatch, and residual aortic regurgitation.
Table 1 lists the documented complications that are caused by a short annulus area, low coronary height, or low sinus of Valsalva. It also represents how the THV design characteristics and availability in different sizes of the selected THV can help in overcoming those problems.
We report the successful implantation of balloon-expandable Myval THV in a high-risk severe AS patient with low coronary heights. Although no additional coronary protection strategies were used, all iatrogenic and postprocedural complications including the risk of coronary flow obstruction were avoided. Moreover, annular rupture or paravalvular leak did not occur. Thus, the case presents new evidence of successful coronary protection in a high-risk patient, whose thorough MS-CT assessment of aortic root enabled the implantation of appropriate THV size with accurate positioning. The procedure was completed without any percutaneous revascularization and the selected THV having the lowest frame height available helped in avoiding in-hospital mortality. Since this is a single case of TAVR in a patient with anatomical complexity, larger studies would be necessary to assess the evolvements in this critical indication for TAVR.
Ethics statement
The patient provided written informed consent prior to the procedure. No identifiable images have been used. The local ethics committee approved the publication of the case findings as per the review of the institutional review board.
- Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, Zühlke L, Prendergast BD. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021 Dec;18(12):853-864. doi: 10.1038/s41569-021-00570-z. Epub 2021 Jun 25. PMID: 34172950.
- Aluru JS, Barsouk A, Saginala K, Rawla P, Barsouk A. Valvular Heart Disease Epidemiology. Med Sci (Basel). 2022 Jun 15;10(2):32. doi: 10.3390/medsci10020032. PMID: 35736352; PMCID: PMC9228968.
- Santangelo G, Bursi F, Faggiano A, Moscardelli S, Simeoli PS, Guazzi M, Lorusso R, Carugo S, Faggiano P. The Global Burden of Valvular Heart Disease: From Clinical Epidemiology to Management. J Clin Med. 2023 Mar 10;12(6):2178. doi: 10.3390/jcm12062178. PMID: 36983180; PMCID: PMC10054046.
- Hanzel GS. Transcatheter aortic valve replacement. In: Aortic Stenosis: Case-Based Diagnosis and Therapy. Springer-Verlag London Ltd. 2015; 253–69.
- Tam DY, Azizi PM, Fremes SE, Chikwe J, Gaudino M, Wijeysundera HC. The cost-effectiveness of transcatheter aortic valve replacement in low surgical risk patients with severe aortic stenosis. Eur Heart J Qual Care Clin Outcomes. 2021 Oct 28;7(6):556-563. doi: 10.1093/ehjqcco/qcaa058. PMID: 32645146.
- Boskovski MT, Nguyen TC, McCabe JM, Kaneko T. Outcomes of Transcatheter Aortic Valve Replacement in Patients with Severe Aortic Stenosis: A Review of a Disruptive Technology in Aortic Valve Surgery. JAMA Surgery. American Medical Association. 2020; 155:69–77.
- Kim H, Lee SJ, Hong SJ, Shim CY, Ahn CM, Kim JS, Kim BK, Hong GR, Ko YG, Choi D, Jang Y, Hong MK. Clinical Outcomes of Transcatheter Aortic Valve Implantation for Native Aortic Valves in Patients with Low Coronary Heights. Yonsei Med J. 2021 Mar;62(3):209-214. doi: 10.3349/ymj.2021.62.3.209. PMID: 33635010; PMCID: PMC7934105.
- Sengottuvelu G, Kumar V, Seth A. The Myval Transcatheter Heart Valve System for the Treatment of Severe Aortic Stenosis – Current Evidence and Future Directions. Heart Int. 2020;14(2):86. https://www.touchcardio.com/interventional-cardiology/journal-articles/the-myval-transcatheter-heart-valve-system-for-the-treatment-of-severe-aortic-stenosis-current-evidence-and-future-directions/
- Elkoumy A, Jose J, Terkelsen CJ, Nissen H, Gunasekaran S, Abdelshafy M, Seth A, Elzomor H, Kumar S, Bedogni F, Ielasi A, Dora SK, Chandra S, Parikh K, Unic D, Wijns W, Baumbach A, Mylotte D, Serruys P, Soliman O. Safety and Efficacy of Myval Implantation in Patients with Severe Bicuspid Aortic Valve Stenosis-A Multicenter Real-World Experience. J Clin Med. 2022 Jan 15;11(2):443. doi: 10.3390/jcm11020443. PMID: 35054137; PMCID: PMC8779274.
- Amat-Santos IJ, García-Gómez M, de Marco F, Won-Keun K, Brito J, Halim J, Jose J, Sengotuvelu G, Seth A, Terkelsen C, Protasiewicz M, Bonilla N, García B, Sánchez-Luna JP, Blasco-Turrión S, González JC, González-Bartol E, Ijsselmuiden AJJ, Gómez-Salvador I, Carrasco Moraleja M, San Román A. Latest-iteration balloon- and self-expandable transcatheter valves for severe bicuspid aortic stenosis: the TRITON study. Rev Esp Cardiol (Engl Ed). 2023 Mar 9:S1885-5857(23)00067-1. English, Spanish. doi: 10.1016/j.rec.2023.03.002. Epub ahead of print. PMID: 36898524.
- Barki M, Ielasi A, Buono A, Maliandi G, Pellicano M, Bande M, Casilli F, Messina F, Uccello G, Briguglia D, Medda M, Tespili M, Donatelli F. Clinical Comparison of a Novel Balloon-Expandable Versus a Self-Expanding Transcatheter Heart Valve for the Treatment of Patients with Severe Aortic Valve Stenosis: The EVAL Registry. J Clin Med. 2022 Feb 12;11(4):959. doi: 10.3390/jcm11040959. PMID: 35207232; PMCID: PMC8876233.
- García-Gómez M, Delgado-Arana JR, Halim J, De Marco F, Trani C, Martin P, Won-Keun K, Montorfano M, den Heijer P, Bedogni F, Sardella G, IJsselmuiden AJJ, Campante Teles R, Aristizabal-Duque CH, Gordillo X, Santos-Martinez S, Barrero A, Gómez-Salvador I, Ancona M, Redondo A, Román JAS, Amat-Santos IJ. Next-generation balloon-expandable Myval transcatheter heart valve in low-risk aortic stenosis patients. Catheter Cardiovasc Interv. 2022 Feb;99(3):889-895. doi: 10.1002/ccd.29923. Epub 2021 Aug 14. PMID: 34390296.
- Kawashima H, Wang R, Mylotte D, Jagielak D, De Marco F, Ielasi A, Onuma Y, den Heijer P, Terkelsen CJ, Wijns W, Serruys PW, Soliman O. Quantitative Angiographic Assessment of Aortic Regurgitation after Transcatheter Aortic Valve Implantation among Three Balloon-Expandable Valves. Glob Heart. 2021 Mar 19;16(1):20. doi: 10.5334/gh.959. PMID: 33833944; PMCID: PMC7977026.
- Santos-Martinez S, Halim J, Castro-Mejía A, De Marco F, Trani C, Martin P, Infusino F, Ancona M, Moreno R, den Heijer P, Nombela-Franco L, Bedogni F, Sardella G, Montorfano M, Revilla-Orodea A, Delgado-Arana JR, Barrero A, Gómez-Salvador I, IJsselmuiden AJJ, Redondo A, Gutiérrez H, Serrador A, Serruys PW, Román JAS, Amat-Santos IJ. Myval versus alternative balloon- and self-expandable transcatheter heart valves: A central core lab analysis of conduction disturbances. Int J Cardiol. 2022 Mar 15;351:25-31. doi: 10.1016/j.ijcard.2021.12.049. Epub 2022 Jan 1. PMID: 34979152.
- Gupta P, Arora S, Qamar A, Gupta M, Seth A. Current status of transcatheter aortic valve replacement in India. Cardiovasc Diagn Ther. 2020 Feb;10(1):83-88. doi: 10.21037/cdt.2019.05.04. PMID: 32175230; PMCID: PMC7044098.
- Sharma SK, Rao RS, Chandra P, Goel PK, Bharadwaj P, Joseph G, Jose J, Mahajan AU, Mehrotra S, Sengottovelu G, Ajit Kumar VK, Manjunath CN, Abhaichand RK, Sethi R, Seth A; Collaborators. First-in-human evaluation of a novel balloon-expandable transcatheter heart valve in patients with severe symptomatic native aortic stenosis: the MyVal-1 study. EuroIntervention. 2020 Aug 28;16(5):421-429. doi: 10.4244/EIJ-D-19-00413. Erratum in: EuroIntervention. 2020 Aug 28;16(5):429. PMID: 31566572.
- Todaro D, Picci A, Barbanti M. Technical characteristics and evidence to date for FDA-and CE Mark-approved valves. 2017; 11.
- Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012 May 3;366(18):1696-704. doi: 10.1056/NEJMoa1202277. Epub 2012 Mar 26. Erratum in: N Engl J Med. 2012 Aug 30;367(9):881. PMID: 22443478.
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Feb 2;143(5):e72-e227. doi: 10.1161/CIR.0000000000000923. Epub 2020 Dec 17. Erratum in: Circulation. 2021 Feb 2;143(5):e229. Erratum in: Circulation. 2023 Aug 22;148(8):e8. PMID: 33332150.
- Nakashima M, Watanabe Y. Transcatheter Aortic Valve Implantation in Small Anatomy: Patient Selection and Technical Challenges. Interv Cardiol. 2018 May;13(2):66-68. doi: 10.15420/icr.2017:28:1. PMID: 29928310; PMCID: PMC5980651.
- Conzelmann LO, Würth A, Schymik G, Schröfel H, Anusic T, Temme S, Tzamalis P, Gerhardus J, Mukherjee C, Gonska BD, Schmitt C, Mehlhorn U. Feasibility of transcatheter aortic valve implantation in patients with coronary heights ≤7 mm: insights from the transcatheter aortic valve implantation Karlsruhe (TAVIK) registry. Eur J Cardiothorac Surg. 2018 Oct 1;54(4):752-761. doi: 10.1093/ejcts/ezy130. PMID: 29617804.
- Yamamoto M, Shimura T, Kano S, Kagase A, Kodama A, Koyama Y, Watanabe Y, Tada N, Takagi K, Araki M, Shirai S, Hayashida K. Impact of preparatory coronary protection in patients at high anatomical risk of acute coronary obstruction during transcatheter aortic valve implantation. Int J Cardiol. 2016 Aug 15;217:58-63. doi: 10.1016/j.ijcard.2016.04.185. Epub 2016 May 4. PMID: 27179209.
- Akinseye OA, Jha SK, Ibebuogu UN. Clinical outcomes of coronary occlusion following transcatheter aortic valve replacement: A systematic review. Cardiovasc Revasc Med. 2018 Mar;19(2):229-236. doi: 10.1016/j.carrev.2017.09.006. Epub 2017 Sep 12. PMID: 29102344.
- Lee CH, Inohara T, Hayashida K, Park DW. Transcatheter Aortic Valve Replacement in Asia: Present Status and Future Perspectives. JACC Asia. 2021 Dec 7;1(3):279-293. doi: 10.1016/j.jacasi.2021.10.006. PMID: 36341218; PMCID: PMC9627874.
- Watanabe Y, Hayashida K, Takayama M, Mitsudo K, Nanto S, Takanashi S, Komiya T, Kuratani T, Tobaru T, Goto T, Lefèvre T, Sawa Y, Morice MC. First direct comparison of clinical outcomes between European and Asian cohorts in transcatheter aortic valve implantation: the Massy study group vs. the PREVAIL JAPAN trial. J Cardiol. 2015 Feb;65(2):112-6. doi: 10.1016/j.jjcc.2014.05.001. Epub 2014 Jun 11. PMID: 24927855.
- Yoon SH, Ohno Y, Araki M, Barbanti M, Lin MS, Ahn JM, Yang DH, Kim YH, Immé S, Gulino S, Tamburino CI, Sgroi C, Park DW, Kang SJ, Lee SW, Lee CW, Park SW, Muramatsu T, Kao HL, Tamburino C, Park SJ. Comparison of aortic root anatomy and calcification distribution between Asian and Caucasian patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2015 Nov 15;116(10):1566-73. doi: 10.1016/j.amjcard.2015.08.021. Epub 2015 Aug 31. PMID: 26428022.
- Khan JM, Kamioka N, Lisko JC, Perdoncin E, Zhang C, Maini A, Chen M, Li Y, Ludwig S, Westermann D, Amat Santos IJ, Kalińczuk Ł, Sinning JM, Kawaguchi T, Fuku Y, Cheema AN, Félix-Oliveira A, Yamamoto M, Kagase A, Codner P, Valle RD, Iyer VS, Kim HS, Lin MS, Maini B, Rodriguez R, Montorfano M, Ancona MB, Tada N, Miyasaka M, Ahmad H, Ruggiero NJ, Torguson R, Ben-Dor I, Shults CC, Weissman G, Lederman RJ, Greenbaum AB, Babaliaros VC, Waksman R, Rogers T. Coronary Obstruction From TAVR in Native Aortic Stenosis: Development and Validation of Multivariate Prediction Model. JACC Cardiovasc Interv. 2023 Feb 27;16(4):415-425. doi: 10.1016/j.jcin.2022.11.018. PMID: 36858660; PMCID: PMC9991077.
- Delgado-Arana JR, Gordillo-Monge MX, Halim J, De Marco F, Trani C, Martin P, Infusino F, Ancona M, den Heijer P, Bedogni F, Nombela Franco L, Moreno R, Sargella G, Montorfano M, Aristizabal-Duque C, Romero-Delgado T, Santos S, Barrero A, Gomez Salvador I, IJsselmuiden S, Redondo Diéguez A, San Román Calvar JA, Amat-Santos IJ. Early clinical and haemodynamic matched comparison of balloon-expandable valves. Heart. 2022 May;108(9):725-732. doi: 10.1136/heartjnl-2021-319349. Epub 2021 Jul 20. PMID: 34285104.
- Lerakis S, Hayek SS, Douglas PS. Paravalvular aortic leak after transcatheter aortic valve replacement: current knowledge. Circulation. 2013 Jan 22;127(3):397-407. doi: 10.1161/CIRCULATIONAHA.112.142000. PMID: 23339094.