More Information
Submitted: December 13, 2023 | Approved: December 27, 2023 | Published: December 28, 2023
How to cite this article: Magdy G, Azab SH, Esmail YA, Elfaky MK. Value of Speckle Tracking Echocardiography in Prediction of Left Ventricular Reverse Remodeling in Patients with Chronic total Occlusion Undergoing Percutaneous Coronary Interventions. J Cardiol Cardiovasc Med. 2023; 8: 164-170.
DOI: 10.29328/journal.jccm.1001170
Copyright License: © 2023 Magdy G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chronic total occlusion; Left ventricular reverse remodeling; Speckle tracking Echocardiography
Value of Speckle Tracking Echocardiography in Prediction of Left Ventricular Reverse Remodeling in Patients with Chronic total Occlusion Undergoing Percutaneous Coronary Interventions
Gehan Magdy*, Sahar Hamdy Azab, Yasmin Ali Esmail, Mohamed Khalid Elfaky
Department of Cardiology and Angiology, Faculty of Medicine, University of Alexandria, Egypt
*Address for Correspondence: Gehan Magdy, Department of Cardiology and Angiology, Faculty of Medicine, University of Alexandria, Egypt, Email: [email protected]
Background: Revascularization procedures for chronic complete occlusion (CTO) are technically challenging but aim to improve left ventricular (LV) function. The aim of this study is to evaluate the value of global longitudinal strain (GLS) measured by 2D-speckle tracking echocardiography( 2D-STE) in the assessment of LV reverse remodeling in patients with CTO undergoing revascularization by percutaneous coronary intervention (PCI).
Methods: Our study included 54 patients with CTO treated by PCI. We evaluate LV systolic function by measurement of left ventricular ejection fraction (LVEF), left ventricular end-systolic volume (LVESV), and the GLS within 24 hours before the PCI and after 3 months post-procedure.
Results: The mean age of the patients was 56.65 ± 7.65 years; 74.1% were males. There was a significant improvement in the LVESV (p < 0.001), LVEF (p < 0.001), and GLS (p < 0.001) at 3 months post-PCI, and by multivariate regression analysis, the GLS was the single most significant predictor of LV reverse remodeling post revascularization (p < 0.001).
Conclusion: Revascularization of coronary CTO lesions by PCI is associated with a significant improvement in regional and global LV function. The GLS measured by 2D-STE is a strong predictor of LV reverse remodeling post-CTO interventions.
HbA1c: Glycated Hemoglobin; 2D-STE: Two-Dimensional Speckle Tracking Echocardiography; ACS: Acute Coronary Syndrome; CABG: Coronary Artery Bypass Graft Surgery; CAD: Coronary Artery Disease; CCS: Canadian Cardiovascular Society; CTO: Chronic Total Occlusion; DM: Diabetes Mellitus; E: Early Mitral Inflow Velocity; e’: Mitral Annulus Early Diastolic Velocity; ECG: Electrocardiogram; eGFR: Estimated Globular Filtration Rate; GLS: Global Longitudinal Strain; HDL: High-Density Lipoprotein Cholesterol; IHD: Ischemic Heart Disease; J: Score-Japanese Score; LAD: Left Anterior Descending Coronary Artery; LAVI: Left Atrial Volume Index; LBBB: Left Bundle Branch Block; LCX: Left Circumflex Coronary Artery; LDL: Low-Density Lipoprotein Cholesterol; LV: Left Ventricle; LVEDV: Left Ventricular End-Diastolic Volume; LVEF: Left Ventricular Ejection Fraction; LVESV: The Left Ventricular End-Systolic Volume; NSTEMI: Non-ST-Segment Elevation Myocardial Infarction; PCI: Percutaneous Coronary Interventions; RCA: Right Coronary Artery; RLS: Regional Longitudinal Strain; ROC: Receiver Operator Characteristic; STEMI: ST-Segment Elevation Myocardial Infarction; SYNTAX: The Synergy between PCI with Taxus and Cardiac Surgery; UA: Unstable Angina
Coronary chronic total occlusion (CTO) is known as 100% obstruction of a coronary artery with thrombolysis in myocardial infarction (TIMI) flow grade 0 lasting more than three months [1,2]. Although technically difficult, revascularization procedures for CTOs are intended to improve the patient’s exercise capacity, patients symptoms, and left ventricular (LV) function, and ultimately decrease the morbidity and the need for coronary artery bypass graft surgery (CABG) [3]. Two-dimensional speckle tracking echocardiography (2D-STE) is becoming a more objective technique to evaluate LV systolic function by measuring active myocardial deformation [4-6]. Applying the global longitudinal strain (GLS) and the regional longitudinal strain (RLS), which have been shown to be more accurate in identifying the LV myocardial ischemia and more reliable than LV ejection fraction (LVEF) in the detection of subclinical recovery of dysfunctional but viable myocardium [7-9], accordingly, the aim of this study is to evaluate the value of GLS measured by 2D-STE in the assessment of LV reverse remodeling in patients with CTO undergoing revascularization by percutaneous coronary intervention (PCI).
Study design
This is a single-center, prospective, and observational study that was conducted from December 2021 until December 2022 in the cardiology department, at Alexandria Main University Hospitals, Alexandria, Egypt, and included 54 patients with angiographic CTO and treated by PCI. The Medical Ethics Committee of the Faculty of Medicine, Alexandria University approved the study protocol (IRB NO: 00012098, FWA NO: 00018699, and serial NO: 0106956). Informed consent was obtained from all participants before enrollment in the study after a full explanation of the purpose and nature of the study.
Inclusion criteria
All patients who had ischemic heart disease (IHD) and were diagnosed with coronary angiography as having CTO of only one of the main coronary territories were included. The IHD diagnosis before coronary angio was either by significant resting 12-lead ECG changes, presence of resting regional wall motion abnormalities upon transthoracic echocardiographic evaluation, or by one of the following stress modalities (myocardial perfusion study using radioactive isotopes, or dobutamine stress echocardiographic studies).
Exclusion criteria
We excluded patients who had poor echocardiographic windows, severe renal impairment (creatinine clearance < 30 ml/min), left bundle branch block (LBBB), atrial fibrillation, cardiac pacemaker, previous CABG, significant valvular lesion, or angiographically significant and intermediate flow-limiting lesions in other vessels.
Methods
All patients included in the study were subjected to the following:
Demographic and medical history evaluation: Including age, sex, history of diabetes mellitus, hypertension, dyslipidemia, cigarette smoking, family history of coronary artery disease (CAD), history of previous acute coronary syndrome (ACS), prior PCI, and angina pain analysis according to the Canadian Cardiovascular Society (CCS) angina classification.
Laboratory evaluation: Including renal functions, complete blood count, lipid profile, and HbA1c. A 12-lead surface electrocardiogram (ECG) was done on all patients to document ST and T-wave changes.
Coronary angiography and PCI: Baseline angiography data included the CTO territory (left anterior descending {LAD}, the left circumflex {LCX}, and the right coronary artery {RCA}), Japanese( J-CTO ) score, collaterals types, scores including Rentrop collaterals classification scores [10], and baseline Synergy between PCI with Taxus and Cardiac Surgery ( SYNTAX) score [11]. Drug-eluting stents (DES) were used in all studied cases, and the PCI procedural details were described, including the following: the PCI technique (antegrade or retrograde), vascular access, drug-coated balloon, pre- and post-balloon dilation, fluoroscopic time, and contrast volume [12].
Transthoracic echocardiography: It was performed in the left decubitus position using an Epiq 7 ultrasound machine (Philips Healthcare, Eindhoven, Netherlands). All the following parameters were measured within 24 hours before PCI and followed up 3 months later:
The left ventricular end-systolic volume (LVESV) and left ventricular end-diastolic volume (LVEDV) were measured and LV ejection fraction (LVEF) was automatically calculated using the biplane area-length method [13].
Diastolic function parameters of the LV were measured, and the E/e′ ratio was calculated to estimate the LV filling pressures, and left atrial volume index (LAVi) was also measured [14].
2D-STE of the LV was done, and the longitudinal strain was evaluated by acquiring cine images from the apical long axis, two- and four-chamber views. These cine images were stored for further offline analysis, where the software’s bull’s-eye method automatically calculated the GLS as the average of the peak systolic longitudinal strain of the 17 LV segments [15].
The regional longitudinal strain (RLS) was calculated for the CTO territory involved, whether it was in the LAD, LCX, or RCA regions. The LV regions were categorized into three areas based on the assessment of collateral circulation in coronary angiography [16].
o The CTO area is referred to as the recipient area.
o The donor area represented the source of collateral blood flow.
o The remaining other regions represent the non-donor/non-CTO area.
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp.) Qualitative data were described using numbers and percentages. Quantitative data were described using the mean and standard deviation. A probability value (p - value) less than 0.05 was considered statistically significant. A paired t-test is used for normally distributed quantitative variables to compare between two periods. The Wilcoxon signed-rank test is used for abnormally distributed quantitative variables to compare between two periods. The Spearman coefficient is used to correlate between two distributed, not normally quantitative variables. The receiver operating characteristic curve (ROC) is generated by plotting sensitivity (TP) on the Y-axis versus 1-specificity (FP) on the X-axis at different cut-off values.
Baseline demographic and laboratory characteristics of the studied patients
The study included 54 patients with a mean age of 56.65 ± 7.65 years; 40 patients (74.1%) were males, and the mean BMI was 27.51 ± 3.51 kg/m2. 35 (64.8%) patients had CCS ≥ 2; the different risk factors and laboratory investigations are mentioned in Table 1. In the Standard 12-ECG, all patients were in normal sinus rhythm, 15 patients (27.8%) had ST segment deviation and 16 patients (29.6%) had T wave changes, which denoted the presence of ischemic changes.
| Table 1: Baseline characteristic of the study population (n = 54). | |
| Demographic data | |
| Age (years )(Mean ± SD) | 56.65 ± 7.65 |
| Male gender (NO %) | 40 (74.1) |
| Risk factor for CAD (NO %) | |
| Systemic arterial hypertension | 28 (51.9) |
| Diabetes mellitus type II | 26 (48.1) |
| Dyslipidemia | 9 (16.7) |
| Smoking | 19 (35.2) |
| Family history of CAD | 4 (7.4) |
| Previous cardiac history (NO %) | |
| CCS≥2 | 35 (64.8) |
| Unstable angina / NSTEMI | 9 (16.6) |
| STEMI | 10 (18.4) |
| PCI | 11 (20.4) |
| Drug history (NO %) | |
| Beta-blockers | 52(96.3) |
| Non-dihydropyridine calcium channel blockers | 2(3.7) |
| Acetyl salicylic acid(ASA) | 24(44.4) |
| ASA+Clopidogrel | 19(35.2) |
| ASA+ticgrelol | 11(20.4) |
| Statins | 40(74.1) |
| Statins + ezetimibe | 14(25.9) |
| ACE or ARBS | 35(64.8) |
| Heart rate(bpm)(mean ± SD) | 76.14 ± 28.91 |
| Systolic BP(mmHg)(mean ± SD) | 125.36 ± 18.11 |
| Diastolic BP(mmHg)(mean ± SD) | 70.33 ± 21.82 |
| Laboratory investigations(mean ± SD) | |
| Glomerular filtration rate (ml/min) | 85.44 ± 12.77 |
| Hemoglobin (mg/dl) | 12.59 ± 1.64 |
| Total cholesterol(mg/dl) | 155.8 ± 39.08 |
| Low density lipoprotein cholesterol(mg/dl) | 99.55 ± 23.02 |
| High density lipoprotein cholesterol (mg/dl) | 58.05 ± 7.94 |
| Triglycerides (mg/dl) | 142.1 ± 61.86 |
| Glycated hemoglobin (%) | 6.22 ± 0.90 |
| ASA: Acetyl Salicylic Acid; BP: Blood Pressure; CAD: Coronary Artery Disease; CCS: Canadian Cardiovascular Society; NSTEMI: Non ST Segment Elevation Myocardial Infarction; STEMI: ST Segment Elevation Myocardial Infarction | |
Baseline coronary angiographic and PCI procedural data
The baseline angiographic data of the 54 studied patients are summarized in Table 2. The most common territory involved in our study is the LAD in 55.6% of our study population, the baseline SYNTAX score was 13.31 ± 3.93, the J-CTO score was 1.28 ± 0.63, collaterals were present in 98.1% of our patients, the most common type of collateral was septal in 53 (98.1%) of patients, and the Rentrop score of the collaterals revealed that 62.9% of patients had a score of 3, and all patients were stented using drug-eluting stents (DESs). The main vascular access was bi-femoral in 45 (83.3%) of patients. The antegrade approach with escalation was used in 88.9% of patients. The average fluoroscopic time was 43.70 ± 10.74 minutes, and the average contrast volume was 332.59 ± 85.65 ml.
| Table 2: Baseline angiographic data of the studied patients. | |
| Patients (NO %) |
|
| CTO territory involved | |
| LAD | 30(55.6) |
| RCA | 18(33.3) |
| LCX | 6(11.1) |
| Collaterals type | |
| Epicardial | 15(27.8) |
| Septal | 53(98.1) |
| Bypass | 4(7.4) |
| Combine types(septal-epicardial) | 12(22) |
| Antegrade approach | |
| Escalation | 48(88.9) |
| Dissection reentry | 2(3.7) |
| Retrogarde approach | |
| Escalation | 10(18.5) |
| Dissection reentry | 2(3.7) |
| Combined techniques | 7(13) |
| Number of stents per patient | |
| 1 | 22(40.7 |
| 2 | 24(44.4) |
| 3 | 8(14.8) |
| Number of balloons pre-dilation | |
| 1 | 35(64.8) |
| 2 | 18(33.3) |
| Number of balloons post-dilation | |
| 1 | 34(63.0) |
| 2 | 8(14.8) |
| CTO: Chronic Total Occlusion; LAD: Left Anterior Descending; LCX: Left Circumflex Artery; RCA: Right Coronary Artery | |
Transthoracic echocardiographic analysis
The LVESV showed a significant decrease (p < 0.001); similarly, the LVEF showed a significant increase (p < 0.001); and the E/e’ ratio showed a significant improvement (p < 0.001) from before to 3 months post CTO –intervention, however, no significant differences were found for LVEDV and LAVI post-intervention, as shown in Table 3.
| Table 3: Echocardiographic data of the studied population before and after CTO interventions. | |||
| Echocardiographic parameter | Before PCI (mean ± SD) |
Follow up (mean ± SD) |
p - value |
| LVEDV (ml) | 97.43 ± 20.48 | 95.83 ± 26.40 | 0.109 |
| LVESV (ml) | 45.20 ± 12.60 | 40.83 ± 16.22 | < 0.001* |
| LVEF (%) | 53.91 ± 7.58 | 57.81 ± 7.82 | < 0.001* |
| LAVI (mL/m2) | 30.24 ± 4.88 | 30.26 ± 3.58 | 0.469 |
| E/e′ ratio | 10.84 ± 3.03 | 9.31 ± 2.67 | < 0.001* |
| GLS (%) | 13.38 ± 4.12 | 15.94 ± 3.92 | < 0.001* |
| RLS in CTO area (%) | 13.82 ± 4.34 | 15.31 ± 4.64 | 0.043* |
| RLS in Donor area (%) | 14.82 ± 3.77 | 14.61 ± 4.16 | 0.724 |
| RLS in Non-CTO area (%) | 15.28 ± 5.19 | 17.29 ± 5.20 | 0.011* |
| CTO: Chronic Total Occlusion; E/e′: Early Mitral Inflow Velocity/Mitral Annulus Early Diastolic Velocity; LAVI: Left Atrial Volume Index; LVEDV: Left Ventricular End Diastolic Volume; LVESV: Left Ventricular End Systolic Volume; LVEF: Left Ventricular Ejection Fraction; GLS: Global Longitudinal Strain; RLS: Regional Longitudinal Strain; *Statistically significant at p ≤ 0.05 | |||
Regarding the GLS of the LV, it showed a significant increase at follow-up compared to before interventions (p < 0.001), as shown in Table 3 and Figure 1.
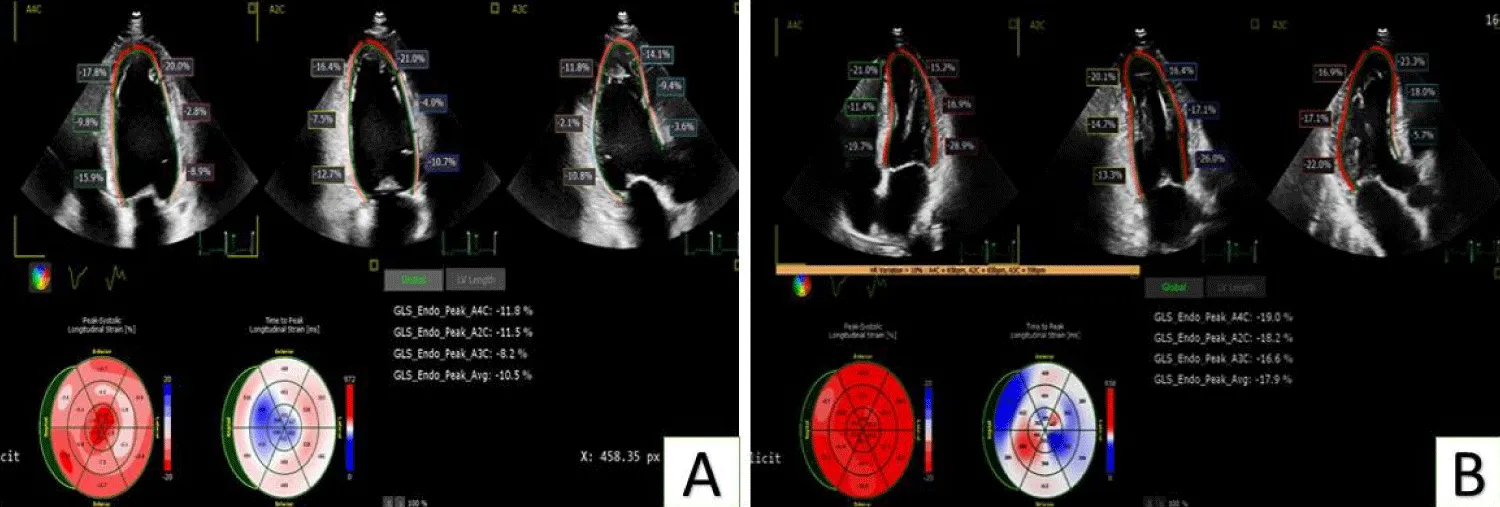
Figure 1: Shows the significant improvement of segmental strain and GLS of a case undergoing PCI to CTO of LCX artery. Image A: before PCI, image B: 3 months post PCI.
As regards the RLS of the LV, the mean RLS in the CTO area and non-CTO area significantly improved 3 months post-CTO interventions (p values of 0.043 and 0.011, respectively), while the mean donor RLS did not significantly improve post-CTO interventions (p = 0.724), as shown in Table 3.
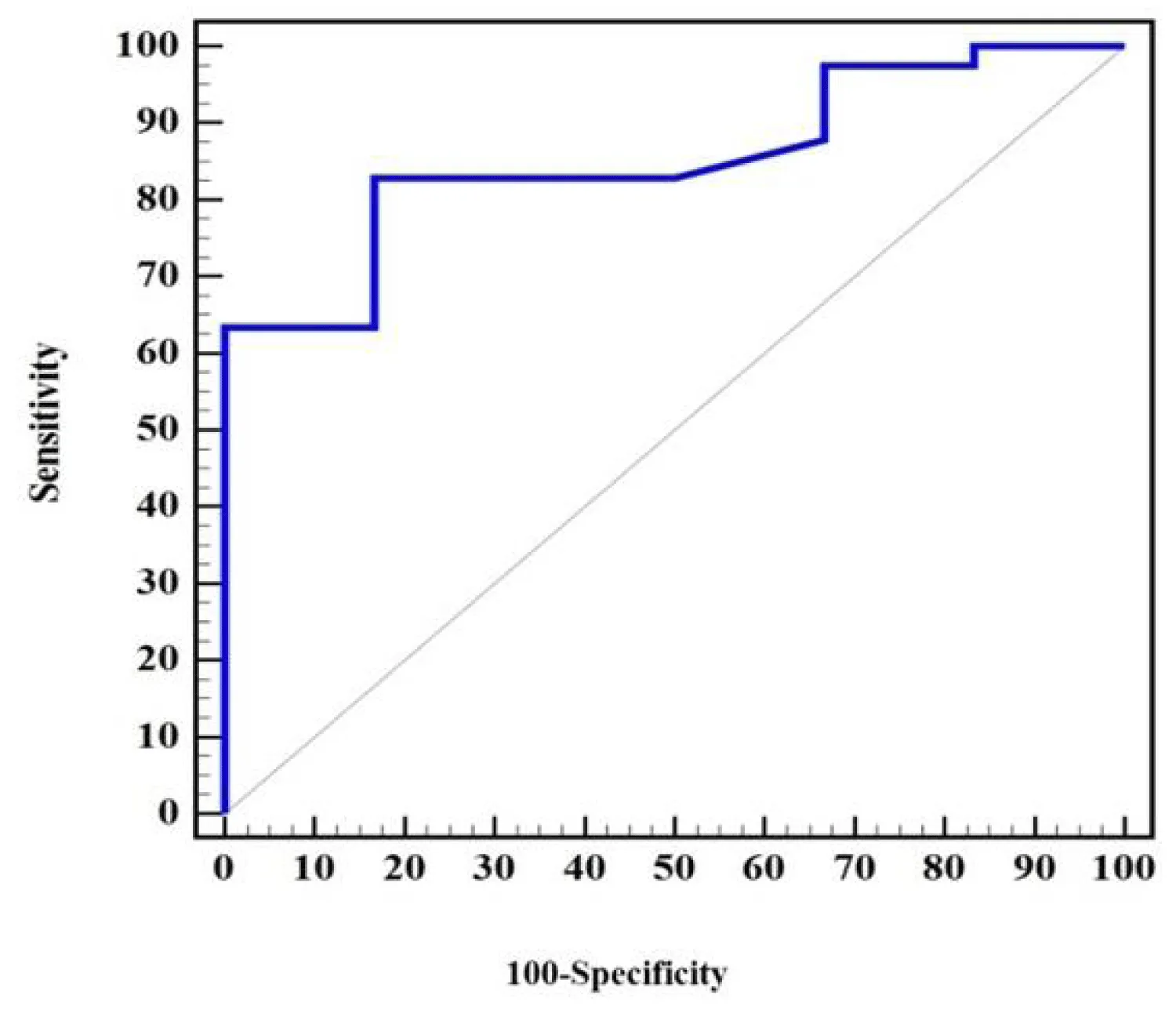
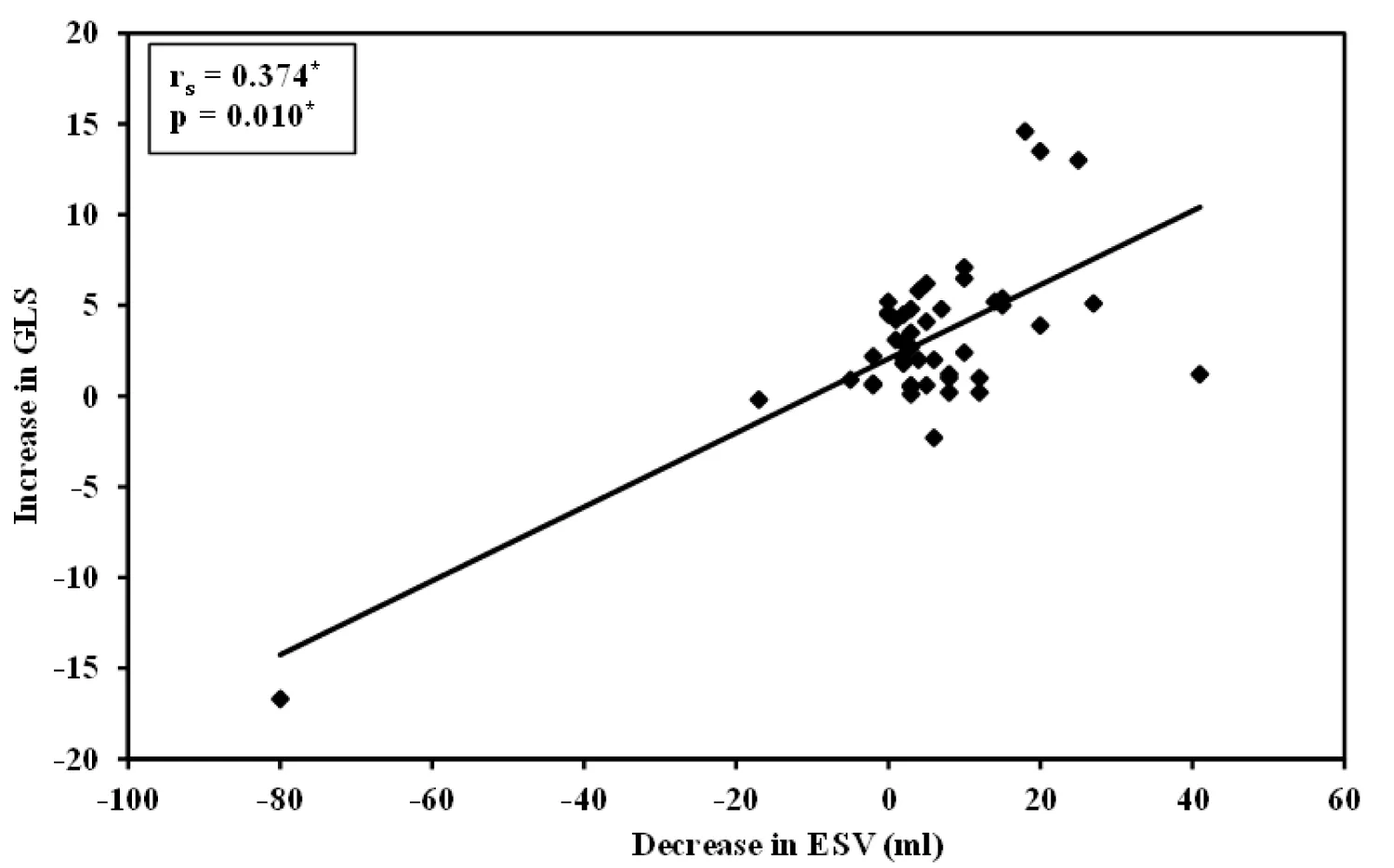
By the ROC curve analysis, GLS can predict reverse LV remodeling, as an increase in GLS by a cut-off value of > 0.9% can predict a decrease of 8.66 ± 9.35 mL of LVESV. (AUC = 0.854, a sensitivity of 82.33, a specificity of 83.33, a PPV = 97.1, and an NPV = 41.7, p = 0.006) as shown in Figure 2 and by correlation coefficient analysis, there is a linearly significant correlation between the increases in GLS and the decrease in LVESV (r = 374; p = 0.010) as shown in Figure 3.
Figure 2: The RCO curve shows the sensitivity and specificity of the GLS to predict improvement in left ventricular end-systolic volume.
Figure 3: Shows a linearly significant correlation between the increases in GLS and the decrease in LVESV.
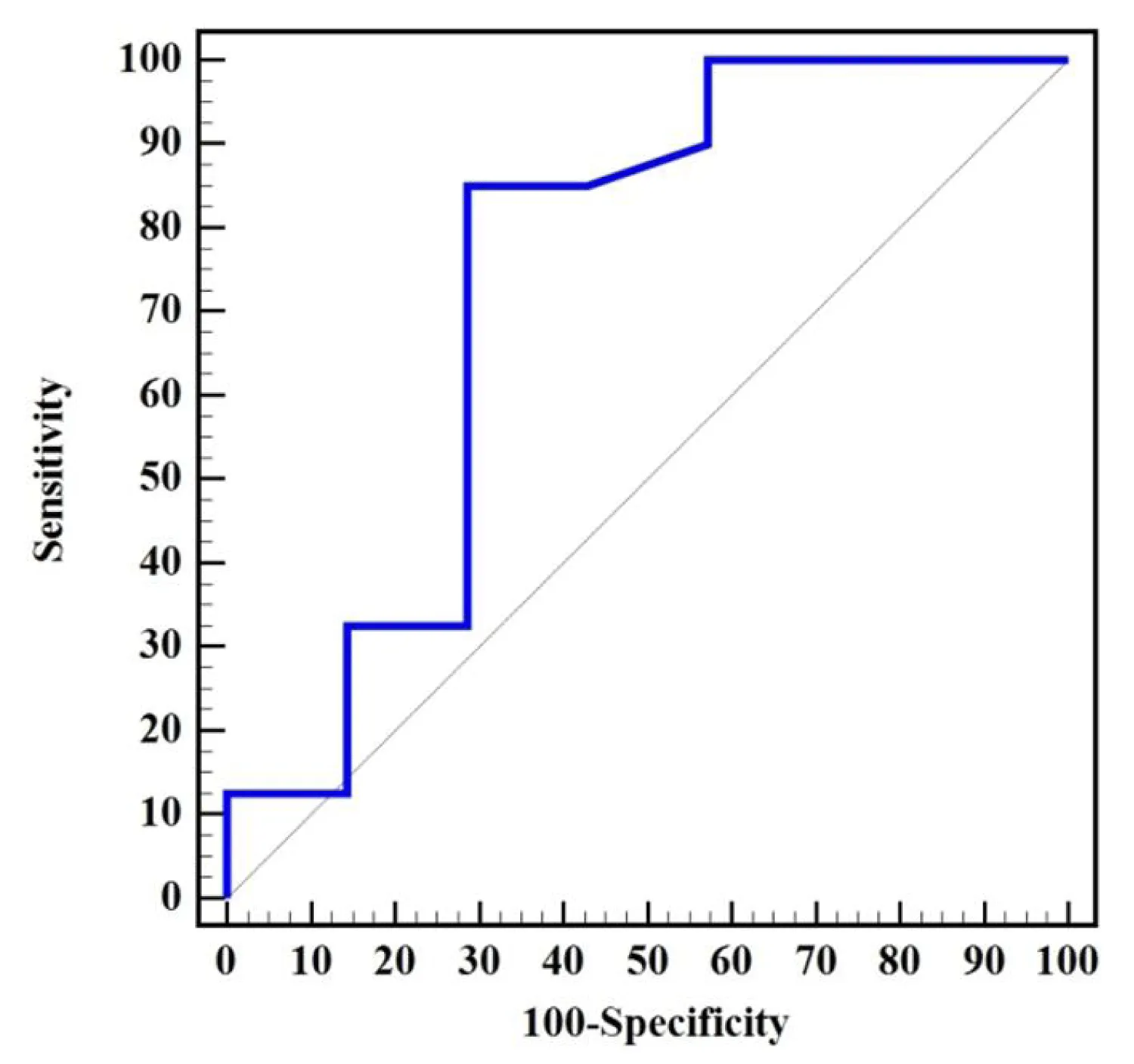
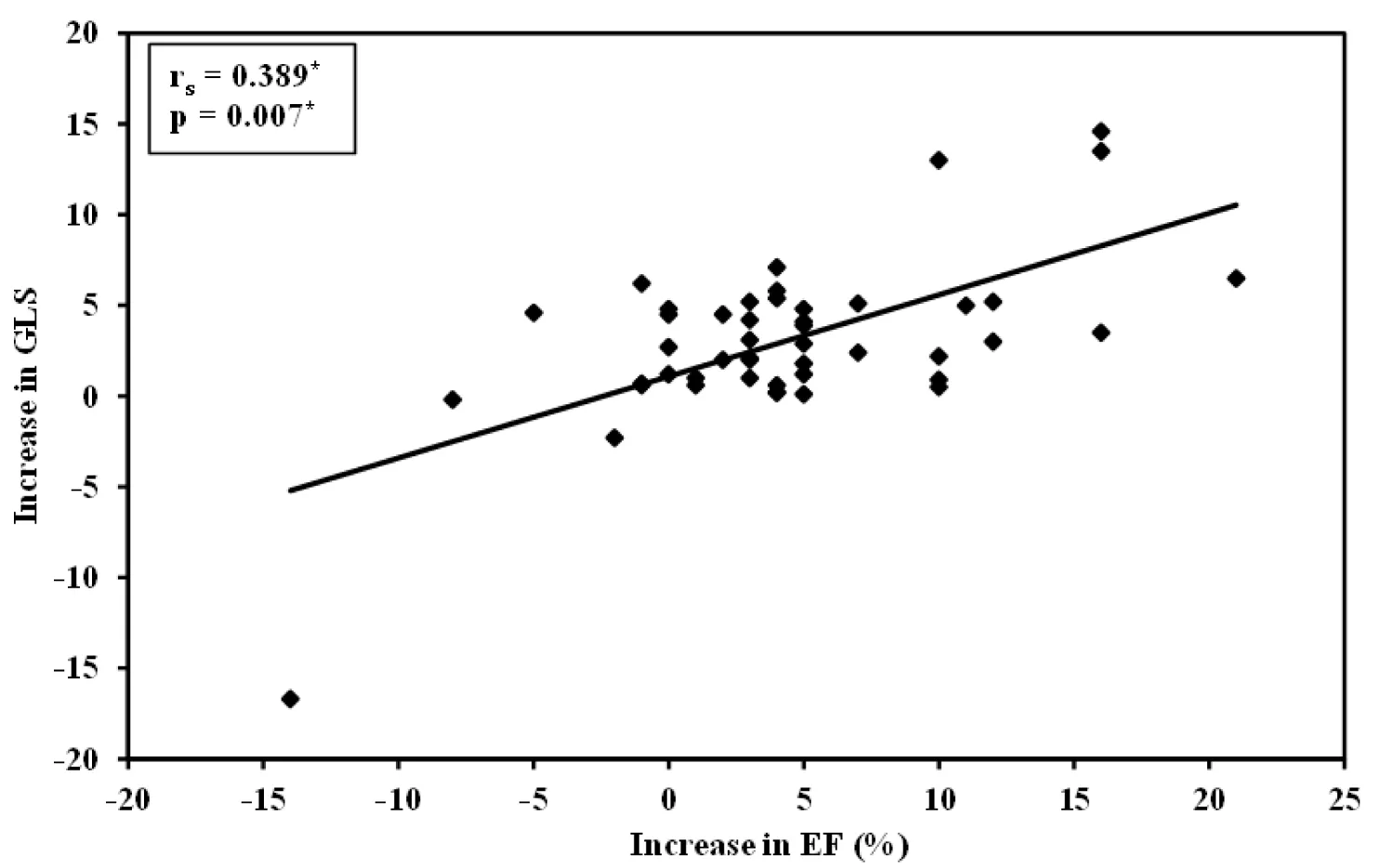
Also, by the ROC curve analysis, GLS can predict reverse LV remodeling, as an increase in GLS by a cutoff value of > 0.7% can predict an increase in LVEF by 5.7% ± 5.64%. (AUC = 0.739, a sensitivity of 85.0, a specificity of 71.43, a PPV = 94.41, and an NPV = 45.5, p = 0.045), as shown in Figure 4, and by correlation coefficient analysis, there is a linearly significant correlation between the increases in GLS and the increase in LVEF (r = 0.389, p = 0.007) as shown in Figure 5.
Figure 4: The RCO curve shows the sensitivity and specificity of the GLS to predict improvement in left ventricular ejection fraction.
Figure 5: Shows a linearly significant correlation between the increases in GLS and the increase in LVEF.
By univariate and multivariate linear regression analysis for the different factors and parameters affecting LV reverse remodeling in patients with CTO undergoing revascularization by PCI, we found that the LVEF and GLS were the significant univariate predictors, p value (0.011, and < 0.001), respectively, and by multivariate analysis, the GLS was the only single predictor of revere remodeling post-PCI (p value < 0.001), as shown in Table 4.
| Table 4: Univariate and multivariate linear regression analysis for the variables affecting left ventricular reverse remodeling in patients with CTO undergoing revascularization by PCI. | ||||
| Univariate | Multivariate | |||
| B (LL – UL 95%C.I) | p | B (LL – UL 95%C.I) | p | |
| Demographic and clinical variables | ||||
| Age | -0.269 (3.341– 2.803) | 0.861 | ||
| Male gender | 0.070 (-0.097– 0.238) | 0.401 | ||
| Hypertension | 0.699 (-1.974– 3.371) | 0.601 | ||
| Diabetes mellitus | -0.189 (-2.874– 2.496) | 0.888 | ||
| Dyslipidemia | 0.574 (-2.827– 3.975) | 0.736 | ||
| Smoking | -1.182 (-4.035– 1.671) | 0.408 | ||
| Family history | -1.002 (-5.795– 3.791) | 0.676 | ||
| CCS | 2.729 (-0.441– 5.899) | 0.090 | ||
| Previous (UA/ NSTEMI) | -1.105 (-3.914– 1.703) | 0.432 | ||
| Previous STEMI | -0.078 (-3.352– 3.196) | 0.962 | ||
| Echocardiographic variables | ||||
| LVEDV | 0.007 (-0.055– 0.070) | 0.820 | ||
| LVESV | 0.082 (-0.017– 0.182) | 0.103 | ||
| LVEF | -0.214 (-0.375 -0.052) | 0.011* | -0.102 (-0.255– 0.051) | 0.185 |
| LAVI | 0.047 (-0.216– 0.311) | 0.719 | ||
| E/e′ ratio | 0.200 (-0.239– 0.639) | 0.363 | ||
| GLS | -0.665 (-0.951– -0.379) | < 0.001* | -0.587 (-0.894– -0.280) | < 0.001* |
| Angiographic variables | ||||
| J-CTO score | 0.639 (-1.463– 2.742) | 0.543 | ||
| Rentrop score | 1.064 (-1.757– 3.886) | 0.451 | ||
| Baseline Syntax score | 0.194 (-0.136– 0.523) | 0.242 | ||
| Antegrade approach | -0.692 (-5.490– 4.105) | 0.773 | ||
| Retrograde approach | -1.856 (-5.083– 1.370) | 0.253 | ||
| CTO -territory involved LAD | 2.084 (-1.427– 5.594) | 0.238 | ||
| CTO-territory involved LCX | 0.330 (-5.151– 5.811) | 0.904 | ||
| CTO-territory involved RCA | 0.330 (-5.151–5.811) | 0.904 | ||
| Fluoroscopic time | -0.008 (-0.133– 0.116) | 0.893 | ||
| Contrast volume | 0.001 (-0.015– 0.018) | 0.874 | ||
| B: Unstandardized Coefficients; C.I: Confidence interval; LL: Lower Limit; UL: Upper Limit; #: All variables with p < 0.05 was included in the multivariate. *: Statistically significant at p ≤ 0.05. |
||||
Myocardial ischemia starts the diastolic dysfunction process and then will be followed by a decrease in LV systolic function. The majority of individuals who have CTO yet have some degree of LV dysfunction, even in the event that coronary collaterals are visible. Although technically challenging, the revascularization process for CTOs attempts to improve survival rates, promote functional recovery, improve symptoms, and decrease morbidity [3], as shown in our patients, who had marvelous improvements in symptoms and quality of life(QOL) post-successful CTO interventions, and this was similar to the results of the study done by Zhao S, et al. [17] who found that during the one-month and one-year follow-ups post-PCI for CTO lesions, symptoms, including dyspnea and angina, significantly improved independent of age (p < 0.05). Similarly, QOL was considerably improved by successful CTO-PCI at the one-month and one-year follow-up (p < 0.01).
2D-STE is becoming a more sensitive and objective technique to evaluate LV systolic and diastolic function [4,5]. Additionally, even in cases where overall LVEF is preserved, myocardial dysfunction can still occur and may be linked to compromised LV longitudinal deformation [6,12].
Successful revascularization to CTO with optimization of the medical therapy had an impact on the improvement of LV function and the reverse of LV remodeling, as seen in our study by the statistically significant improvement in the LVEF by 5.7 ± 5.64% (p < 0.001) and a decrease in the LVESV by 8.66 ± 9.35 ml (p < 0.001) 3 months post successful PCI for CTO, also by ROC curve and correlation coefficient analysis, we found a strong correlation between the percentage of increase in GLS, the percent of improvement in LVEF, and the degree of improvement of LVESV, which explains the predictive value of GLS to predict reverse LV remodeling, and this is in agreement with the meta-analysis done by Michael Megaly, et al. [18] that comprised 34 observational studies revealed that successful CTO PCI was linked to an improvement in LV ejection percent of 4.3% (p < 0.0001), and linked to a 4 mL reduction in LV end-systolic volume (p < 0.0001). Another study was done by Shawky, et al. [19] who studied 100 patients with CTO in the LAD territory, and 2D-STE was performed on days 1 and 3 months later after PCI, they found that GLS, LVEF, and LVESV were significantly improved at 3-month follow-up, which is exactly similar to what we found in our study. Also in the study of P. Wang, et al. [12] studied 43 patients with CTO who had PCI, their LV function was evaluated by 2D-STE and conventional echocardiography after PCI by One day, three months, and six months later, they found that GLS was improved as early as 1 day after PCI; however, the LVEF improved up to 3 and 6 months post-CTO-PCI, which is in agreement with our study in the 3-month follow-up post-PCI improvement in LVEF. However, a study by Chimura, et al. [20] found that nine months after successful PCI, the GLS showed a significant improvement (p < 0.01), while the group that had failed PCI did not significantly change (p = 0.64). However, in both the successful and unsuccessful groups, LVEF and LVESV did not show a significant improvement during the follow-up; their results are not in agreement with our study, as we found that successful PCI to CTO significantly improves LVEF and reduces LVESV.
In our study, we also assessed the effect of a successful CTO revascularization on regional LV function by calculating the RLS, and we found that at 3 months follow up a significant improvement of the RLS of the CTO area and non-CTO area (p values of 0.043 and 0.011, respectively), however, the donor area did not show any significant change (p = 0.724), also Yohei Sotomi, et al. [16] studied a total of 37 patients and 2D-STE was performed before PCI, and after 1-day and 3-months of procedure they found that RLS in CTO and donor areas and GLS were significantly improved 1-day after the procedure, but these improvements diminished during 3 months but their findings are not similar to our study as we found that the improvement of GLS and RLS of CTO area remained significant after 3-months without significant improvement in the donor area.
Study limitations
This study was a single-center study with a small sample size. Another limitation is the lack of comparison to patients who did not undergo intervention or had a failed CTO procedure, which could assess the specific impact of CTO interventions. Also, a prolonged, long-term follow-up beyond the 3 months of our study is recommended.
Revascularization of coronary CTO lesions by PCI is associated with a significant improvement in regional and global LV function. The GLS measured by 2D-STE is a strong predictor of LV reverse remodeling post-CTO interventions.
- Hafeez Y, Varghese V. Chronic Total Occlusion of the Coronary Artery. 2023 Jul 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32809342.
- Galassi AR, Brilakis ES, Boukhris M, Tomasello SD, Sianos G, Karmpaliotis D, Di Mario C, Strauss BH, Rinfret S, Yamane M, Katoh O, Werner GS, Reifart N. Appropriateness of percutaneous revascularization of coronary chronic total occlusions: an overview. Eur Heart J. 2016 Sep 14;37(35):2692-700. doi: 10.1093/eurheartj/ehv391. Epub 2015 Aug 7. PMID: 26254179.
- Olivari Z, Rubartelli P, Piscione F, Ettori F, Fontanelli A, Salemme L, Giachero C, Di Mario C, Gabrielli G, Spedicato L, Bedogni F; TOAST-GISE Investigators. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J Am Coll Cardiol. 2003 May 21;41(10):1672-8. doi: 10.1016/s0735-1097(03)00312-7. PMID: 12767645.
- Ng AC, Tran da T, Newman M, Allman C, Vidaic J, Kadappu KK, Boyd A, Thomas L, Leung DY. Comparison of myocardial tissue velocities measured by two-dimensional speckle tracking and tissue Doppler imaging. Am J Cardiol. 2008 Sep 15;102(6):784-9. doi: 10.1016/j.amjcard.2008.05.027. Epub 2008 Jul 10. PMID: 18774007.
- Blessberger H, Binder T. NON-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart. 2010 May;96(9):716-22. doi: 10.1136/hrt.2007.141002. PMID: 20424157.
- Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004 Oct;17(10):1021-9. doi: 10.1016/j.echo.2004.06.019. PMID: 15452466.
- Dimitriu-Leen AC, Scholte AJ, Katsanos S, Hoogslag GE, van Rosendael AR, van Zwet EW, Bax JJ, Delgado V. Influence of Myocardial Ischemia Extent on Left Ventricular Global Longitudinal Strain in Patients After ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2017 Jan 1;119(1):1-6. doi: 10.1016/j.amjcard.2016.08.091. Epub 2016 Sep 30. PMID: 27776800.
- Liu C, Li J, Ren M, Wang ZZ, Li ZY, Gao F, Tian JW. Multilayer longitudinal strain at rest may help to predict significant stenosis of the left anterior descending coronary artery in patients with suspected non-ST-elevation acute coronary syndrome. Int J Cardiovasc Imaging. 2016 Dec;32(12):1675-1685. doi: 10.1007/s10554-016-0959-0. Epub 2016 Aug 13. PMID: 27522670.
- Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012 Jul;33(14):1716-7. doi: 10.1093/eurheartj/ehs124. Epub 2012 Jun 22. PMID: 22730487.
- Werner GS, Surber R, Kuethe F, Emig U, Schwarz G, Bahrmann P, Figulla HR. Collaterals and the recovery of left ventricular function after recanalization of a chronic total coronary occlusion. Am Heart J. 2005 Jan;149(1):129-37. doi: 10.1016/j.ahj.2004.04.042. PMID: 15660044.
- Kappetein AP, Dawkins KD, Mohr FW, Morice MC, Mack MJ, Russell ME, Pomar J, Serruys PW. Current percutaneous coronary intervention and coronary artery bypass grafting practices for three-vessel and left main coronary artery disease. Insights from the SYNTAX run-in phase. Eur J Cardiothorac Surg. 2006 Apr;29(4):486-91. doi: 10.1016/j.ejcts.2006.01.047. Epub 2006 Feb 23. PMID: 16497510.
- Wang P, Liu Y, Ren L. Evaluation of left ventricular function after percutaneous recanalization of chronic coronary occlusions : The role of two-dimensional speckle tracking echocardiography. Herz. 2019 Apr;44(2):170-174. doi: 10.1007/s00059-017-4663-1. Epub 2018 Jan 16. PMID: 29340717; PMCID: PMC6439137.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015 Jan;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003. PMID: 25559473.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016 Apr;29(4):277-314. doi: 10.1016/j.echo.2016.01.011. PMID: 27037982.
- Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015 Jan;16(1):1-11. doi: 10.1093/ehjci/jeu184. Epub 2014 Dec 18. PMID: 25525063.
- Sotomi Y, Okamura A, Iwakura K, Date M, Nagai H, Yamasaki T, Koyama Y, Inoue K, Sakata Y, Fujii K. Impact of revascularization of coronary chronic total occlusion on left ventricular function and electrical stability: analysis by speckle tracking echocardiography and signal-averaged electrocardiogram. Int J Cardiovasc Imaging. 2017 Jun;33(6):815-823. doi: 10.1007/s10554-017-1064-8. Epub 2017 Jan 13. PMID: 28091872.
- Zhao S, Wang J, Chen Y, Wang W, Hu W, Zou Y, Zhu B, Yang L, Chen G, Yu T, Han P, Ma B, Wang H, Xia C, Wang R, Tan Z, Zhai Z, Li R, Gao H, Lian K, Li C. Improvement of Symptoms and Quality of Life After Successful Percutaneous Coronary Intervention for Chronic Total Occlusion in Elderly Patients. J Am Heart Assoc. 2023 Apr 18;12(8):e029034. doi: 10.1161/JAHA.123.029034. Epub 2023 Apr 7. PMID: 37026557; PMCID: PMC10227266.
- Megaly M, Saad M, Tajti P, Burke MN, Chavez I, Gössl M, Lips D, Mooney M, Poulose A, Sorajja P, Traverse J, Wang Y, Kohl LP, Bradley SM, Brilakis ES. Meta-analysis of the impact of successful chronic total occlusion percutaneous coronary intervention on left ventricular systolic function and reverse remodeling. J Interv Cardiol. 2018 Oct;31(5):562-571. doi: 10.1111/joic.12538. Epub 2018 Jul 4. PMID: 29974508.
- Abdelmoneum MS, Sabry AM, Anis AS, Mansour HAK. Evaluation of Left ventricular Function after Revascularization of Coronary Chronic Total Occlusion using Speckle Tracking Echocardiography. Cardiometry.2022;5:51-59.
- Chimura M, Yamada S, Yasaka Y, Kawai H. Improvement of left ventricular function assessment by global longitudinal strain after successful percutaneous coronary intervention for chronic total occlusion. PLoS One. 2019 Jun 12;14(6):e0217092. doi: 10.1371/journal.pone.0217092. PMID: 31188846; PMCID: PMC6561546.