More Information
Submitted: December 26, 2023 | Approved: January 02, 2024 | Published: January 03, 2024
How to cite this article: Trainini JC, Wernicke M, Beraudo M, Trainini A. The Fulcrum of the Human Heart (Cardiac fulcrum). J Cardiol Cardiovasc Med. 2024; 9: 001-005.
DOI: 10.29328/journal.jccm.1001171
Copyright License: © 2024 Trainini JC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cardiac anatomy; Myocardium; Cardiac fulcrum; Myocardial support
The Fulcrum of the Human Heart (Cardiac fulcrum)
Jorge Carlos Trainini1*, Mario Wernicke2, Mario Beraudo3 and Alejandro Trainini3
1Hospital Presidente Perón, Buenos Aires, Universidad Nacional de Avellaneda, Argentina
2Departamento de Anatomía Patológica, Clínica Güemes, Luján, Buenos Aires, Argentina
3Departamento de Cirugía Cardíaca, Clínica Güemes, Luján, Buenos Aires, Argentina
*Address for Correspondence: Jorge Carlos Trainini, MD, PhD, Hospital Presidente Perón, Buenos Aires, Universidad Nacional de Avellaneda, Argentina, Email: [email protected]
Introduction: The functional anatomy of the myocardium allows envisioning that it initiates and ends at the origin of the great vessels. In our research, we have always considered that it should have a point of attachment to allow its helical rotation to fulfill the fundamental movements of shortening-torsion (systole) and lengthening-detorsion (suction), which once found, was called the cardiac fulcrum.
Materials and methods: A total of 31 hearts, arising from the morgue and slaughterhouse were used: 17 corresponded to bovids and 14 were human. Anatomical and histological studies were performed. The heart was fixed in 10% buffered formalin. Hematoxylin-eosin, Masson’s trichrome staining technique, and 4-micron sections were used for the histological study, and 10 % formalin was used as a buffer.
Results: The anatomical investigations have revealed that all the hearts (bovids and humans) have myocardial support whose histological structure in the analyzed specimens presents with an osseous or chondroid-tendinous character. In this structure, which we have called the cardiac fulcrum, are inserted the myocardial fibers at the origin and end of the band, which correspond to the continuous myocardium coiled as a helix.
Conclusion: This description of the fulcrum would end the problem of lack of support of the myocardium to fulfill its function of suction/ejection.
Myocardial fibers form a continuous muscle that describes double helicoids to form both ventricles [1-3]. This study approach correlates with a cardiac structure presenting the remarkable characteristic of being a suction-ejection pump with the size equivalent to a human fist and an average weight of 270 g, which ejects 4-6 liters/minute at a speed of 300 cm/s, consuming only 10 watts, and working without interruption for 80 years without maintenance, almost without noise, and no smoke. Its work is equivalent to the daily withdrawal of 1 ton of water 1 m deep with a mechanical efficiency (work/energy relationship) of 50%. This allows the ejecting of 70% of the left ventricular volume with only 12% shortening of its contractile unit, the sarcomere [4].
The reason for these investigations was to analyze the possibility of support for the insertion of the myocardium through the study of human and bovine cardiac anatomical pieces. The inevitable question that arises is whether the bands surrounding the ventricles should do so on a rigid fulcrum, as does a tendon using a bone insertion as a lever. Are there any in the heart?
A total of 31 hearts, coming from the morgue and slaughterhouse were used: 17 corresponded to 2-year-old bovids (10 males and 7 females), weighing 800 g - 1000 g and 14 were human (8 males and 6 females), two from 16 and 23-week-old fetuses, three from 5, 10 and 27 week-old infants, one from a 4-year-old boy, one from a 10-year-old boy weighing 116 g and seven from adults weighing 300 g.
Anatomical and histological studies were performed. The heart was fixed in 10% buffered formalin. Hematoxylin-eosin, Masson’s trichrome staining technique, and 4-micron sections were used for the histological study, and 10 % formalin was used as a buffer.
The single continuous and helical myocardium was deployed according to a previously published technique [5]. A fundamental concept at the beginning of deployment must be followed, as any attempt at not respecting the dissection of the axes where the myocardium coils as a helix, elicits the myocardial mass rupture. The concurrence of the cardiac muscle origin and end in the cardiac fulcrum constitutes a meeting point between the right segment and the ascending segment, origin and end of the myocardium. Thus, both ends are situated in the same site, with the origin of the myocardial fibers placed in an anterior plane to those of its end.
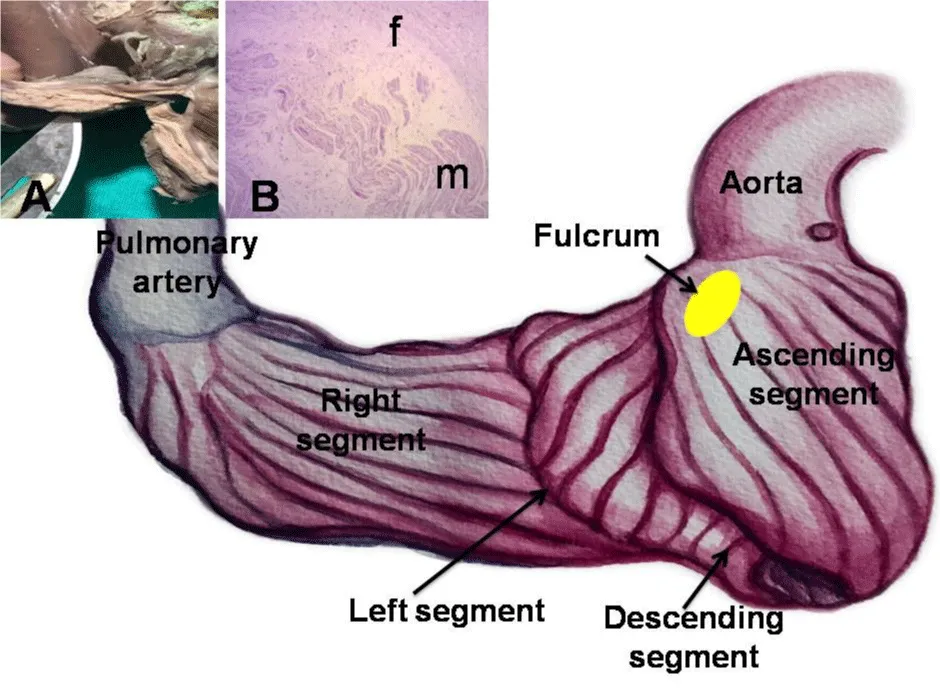
The anatomical investigations have revealed that all the hearts (bovids and humans) have myocardial support whose histological structure in the analyzed specimens presents with an osseous or chondroid-tendinous character. In this structure, which we have called the cardiac fulcrum, are inserted the myocardial fibers at the origin and end of the band, which correspond to the continuous myocardium coiled as a helix (Figures 1,2).
Figure 1: This figure shows the continuous myocardium arrangement at the beginning of its unfolding. The pulmonary artery and the right segment have been separated from the fulcrum in order to indicate its intermediate location between the right segment (anterior location) and the ascending segment (posterior location). A: Macroscopic view of the fulcrum in an adult human heart. B: Microscopic view of the human fulcrum. Note the myocardial fibers inserting into the tendinous fulcrum matrix.
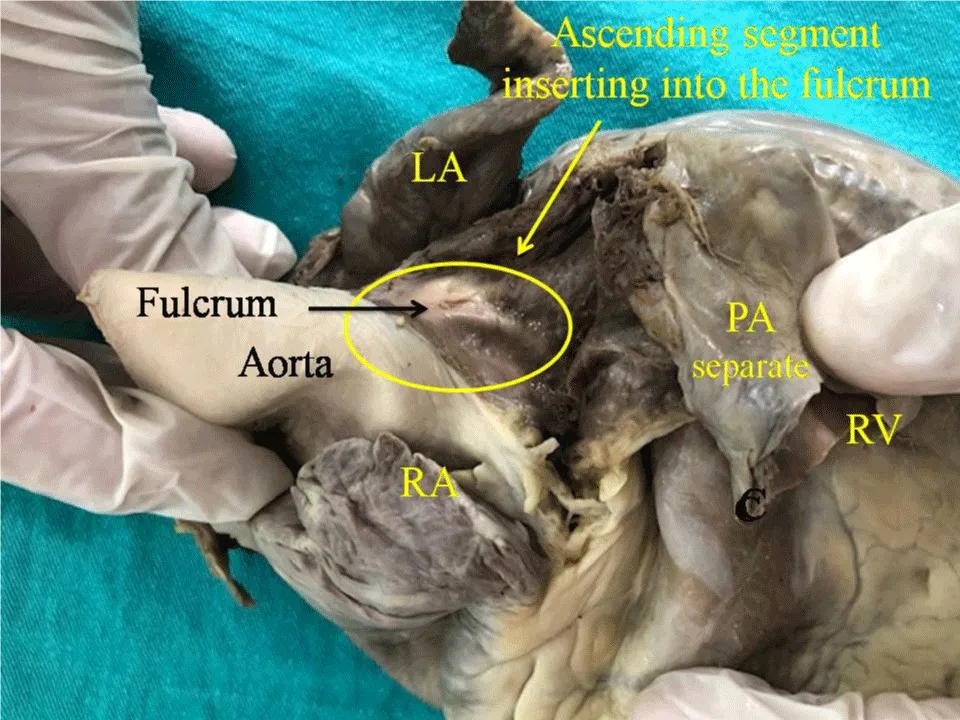
Figure 2: Adult human heart. The ascending segment is seen inserting into the cardiac fulcrum. References: LA: Left Atrium; RA: Right Atrium; PA: Pulmonary Artery; RV: Right Ventricle.
Location and relationships
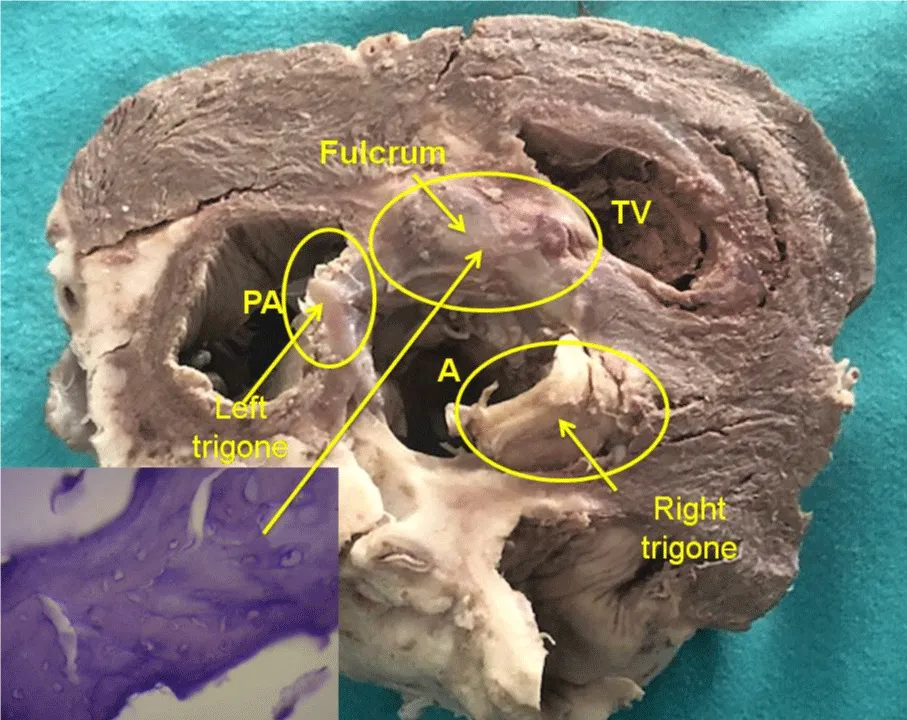
The cardiac fulcrum is found in the proximity of the tricuspid valve (right) the aorta (superior) and the pulmonic-tricuspid cord (anterior) (Figure 3). In order to localize it, it is necessary to shift the pulmonary artery and the right segment to the left of the observer, stripping the aortic root at the origin of the helical myocardium. This maneuver uncovers the fulcrum below the aorta and inferior and to the left of the right trigone, without any continuity with it, below the origin of the right coronary artery, detached from aortic continuity and located as a complementary element between the aorta and the myocardium.
Figure 3: Descriptive photograph of the cardiac fulcrum. PA: Pulmonary Artery; A: Aorta; TV: Tricuspid Valve. In the left bottom can be seen cardiac fulcrum trabecular bone with osteoblasts and segmental lines. The structure makes up the trabecular bone tissue scaffolding similar to the metaphyseal growth area in long bones. The histological section shows bone trabeculae with osteoblasts and segmental lines secondary to bone apposition. Hematoxylin-eosin stain at high magnification (40x). (Transverse section of a bovine heart).
Histology
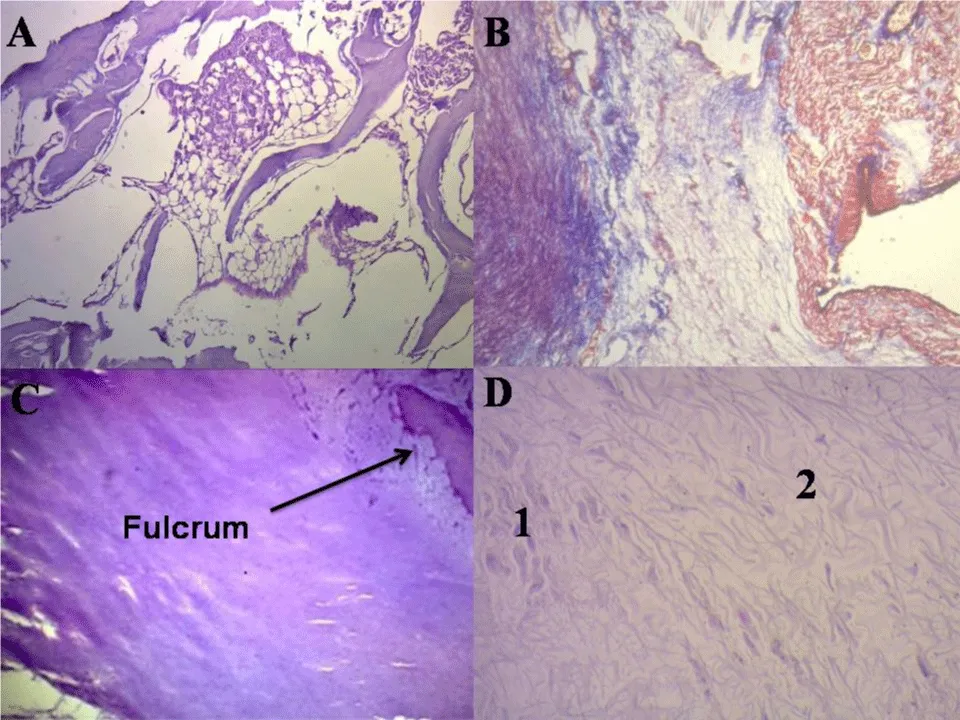
In bovids, the fulcrum is triangular. Its size, corroborated by dissection and imaging techniques, has an average of 37 mm in length, 45 mm in width, and 15 mm in thickness. The microscopic analysis of the bovid cardiac fulcrum shows the presence of osseous trabeculae as a result of endochondral ossification. Its general structure resembles the metaphyseal growth of long bones (Figure 4). It is possible to observe osseous trabeculae with osteoblasts and segmental lines secondary to osseous apposition. The same histological findings have been encountered in the rest of the mammals.
In the 10-year-old human heart, the description of the cardiac fulcrum is related to this early age, as it shows evidence of a central area of the fulcrum formed by chondroid tissue. Given the age, it is logical that its size is smaller. This finding was repeated in the 16 and 23-week-old human fetuses with the characteristic prechondroid bluish areas in a myxoid stroma and in the heart of the 5, 10 and 27-week-old infants (Figure 4).
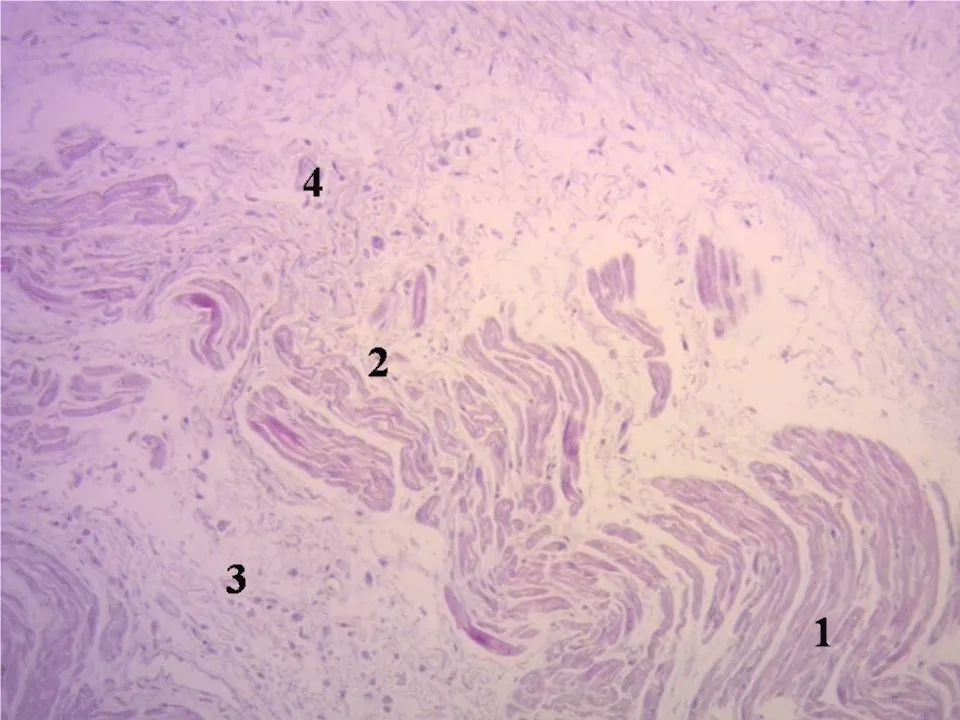
Figure 4: Histology of cardiac fulcrum. A: Mature trabecular bone forming the cardiac fulcrum tissue (bovine heart). Hematoxylin-eosin stain (10x). B: Cardiac fulcrum in a 23-week gestation human fetus showing prechondroid bluish areas in a myxoid stroma. Masson’s Trichrome Technique (15x). C: Central area of the fulcrum formed by chondroid tissue in a10-year-old human heart. Hematoxylin eosin- technique (15x). D: Festooned colagenous bands integrating the fibrotendinous matrix of the fulcrum (adult human heart). Hematoxylin-eosin technique (15x). 1: Remnants of atrophic cardiomyocytes. 2: Fibrotendinous matrix.
The histological analysis of the adult human heart fulcrum (average size of 25 mm in length and 15 mm in width) revealed a chondroid-tendinous matrix. In principle, similar evidence of detection, localization, and morphology of the fulcrum has been found in all the analyzed hearts, both human and bovid (Figure 4). They present myocardial insertion in the rigid fulcrum structure, forming a cardiomyocytic matrix unit, independently of their osseous, cartilaginous, or tendinous nature in the different specimens studied. This point of attachment implies exerting as in any muscle the supporting function of the muscular lever and also acting as a bearing or pad, preventing the ventricular rotation force, either by torque or torsion effort is transferred to the aorta. Thus, it dissipates the energy produced by the helical muscle movement and avoids the strangulation or bending of the artery during the systolic ejective period.
Insertion
The histology of the fulcrum, of collagen-cartilaginous nature, determines the need for an additional analysis to understand its function. In every heart and in all the histological analyses we have verified the insertion of the myocardium in the fulcrum, a finding that becomes a strong point of reasoning to understand the function of the cardiac fulcrum in its biomechanical action of torsion-detorsion. In all the hearts analyzed, we found this attachment of the myocardium in the rigid structure of the fulcrum according to the studies carried out during this investigation (Figure 5).
Figure 5: Festooned cardiomyocytes penetrating the fibrocollagenous matrix. (Cardiac fulcrum). Adult human heart. Hematoxylin-eosin technique (15x). 1: Fibrocollagenous matrix. 2: Cardiomyocyte fraying. 3: Atrophic cardiomyocytes. 4: Festooned cardiomyocytes.
To establish the identity of the cardiac fulcrum, a histological analysis was also performed on the trigones, trying to find cardiomyocytes in their composition as a possible insertion of the cardiac muscle in these structures. Our research only showed collagenous tissue without cardiomyocytes in the trigones, confirming that the fulcrum is the support of the myocardium, both at its origin and end.
Myocardial fibers constitute a single and continuous muscle that describes a double helix to form the walls of both ventricles [6,7]. In order to fulfill its torsion-detorsion muscular function, it needs a point of support that we have found and called the cardiac fulcrum, to which it is attached at its origin and end, similarly to any other muscle [8]. At this point there is an analogy between the skeletal muscle and the myocardium. The former performs its contraction between a fixed and a mobile point of support. This situation is found in the continuous myocardium, as there is greater solidity in the insertion between the fulcrum and the ascending segment in relation to the initial attachment of the right segment in that support [9-11].
From this experience, fundamental questions arise: why have we found that in fetus, infant, child, and adult human hearts the cardiac fulcrum has cartilaginous characteristics, beyond it fulfills the same function of attaching the helical myocardium that other species have? Let us bear in mind that the cartilage is the substrate for endochondral ossification, and although it does not always ossify, it is necessary to that end. Our interpretation is that perhaps the cardiac fulcrum with an osseous characteristic -as observed in bovids- is a vestigial organ typical of the evolution of mammals. A vestigial structure must be understood as the preservation during the “evolutionary” process of genetically established attributes that have lost all or part of their ancestral function in certain species. In this case, the osteo-cartilaginous histology identified in bovids refers to a cartilage-tendinous matrix that is sufficient to achieve myocardial insertion and attain a muscle power that is much lower than that of larger mammals [12,13]. Let us recall that in this investigation, the bovid fulcrum is of osseus nature.
In such a way that the histological analysis of the fulcrum in adult human hearts showed a collagenous matrix of a tendinous Natura, which determines the need for additional elucidation. The fact that it has been found in humans and different species implies that from a functional point of view, its presence is synonymous with the insertion of the myocardium, a fact that we have corroborated in all the histology analyzed, constituting a solid point of reasoning to comply with its biomechanical action.
We find this demonstration when we direct the histological analysis to the insertion point of the myocardium with the cardiac fulcrum, be it bone, chondroid, or tendon. In all the hearts analyzed, this tethering of the myocardium to the rigid structure of the fulcrum was found, integrating a myocardiocytic-matrix unit, even if it was bony, cartilaginous, or tendon according to the studies we have carried out in this investigation.
The important fact that establishes the attachment of myocardial fibers to the cardiac fulcrum stems from both macroscopic and microscopic observation. Its conformation was confirmed by histology. We have called this structure, origin, and end of the helical continuous myocardium, cardiac fulcrum, as a parallelism and tribute to the definition of the point of support acting as a lever expressed by Archimedes of Syracuse [14-18]. It should be noted that in order to visualize the cardiac fulcrum it is essential to deploy the helical myocardium. Figure 3 shows the location of the cardiac fulcrum in the human adult heart.
In 1669, Richard Lower considered that the myocardium was subjected to a torsional movement associated with the helical arrangement of its fibers [19]. He expressed that the heart exerted a movement similar to “wringing a towel.” This concept was later verified in mice by Henson, and more recently in humans through electrophysiological and imaging studies. The heart achieves the ejection of its content through the torsion of its walls and initiates its filling by generating a negative pressure through detorsion. The synchronous torsional movement with longitudinal ventricular shortening can be explained by the helical arrangement and continuity of the cardiac muscle.
The spatial arrangement of the continuous helical myocardium clearly indicates that the propulsion is given by its walls in the ventricular cavities that delimit these structures. Formed by the basal loops (right and left segments) and the apical loop (descending and ascending segments), the muscular unit they form are the walls of the ventricles, to which it provides propulsion power.
Muscle homogenization masks the real spiral continuity of the fibers by the overlapping of its segments. Even the transverse interconnections between the tracts do not invalidate the concept of continuous myocardium, understanding that this compact arrangement is the result of the evolutionary development to obtain solidity in its structure in a strict relationship to its function. This implies considering that its structural strength is required in birds and mammals to ensure that blood is ejected at a high speed during a limited time, by an organ that must supply two circulations (systemic and pulmonary). The anatomical investigation of the heart through an adequate dissection, histological examination [3], imaging analysis of echocardiographic studies, and electrophysiological studies [20-22] carried out with three-dimensional electroanatomical mapping and diffusion tensor cardiac magnetic resonance imaging show the continuous muscular course that circumscribes the two ventricles [23,24].
The spatial helical arrangement of the myocardium forces the muscle to overlap segments [25]. This anatomical situation has a profound correspondence with myocardial movements and with the stimulation that runs through its segments, according to the electrophysiological studies that we have previously carried out.
Function leads the myocardium to have a point of support as any skeletal muscle, both at its origin and end. If the myocardium did not have this helical spatial anatomical conformation, with an insertion at both ends in the cardiac base remaining free at the apex, that is, as a pendulum in the thorax; and if it did not present a stimulation allowing torsion and detorsion, it would be unable to fulfill its extraordinary muscular power. This cardiac fulcrum has been discovered by Jorge Trainini [14-17] and supported by other publications [12,18].
Having found an osseous structure in the bovid cardiac fulcrum and its relationship with the myxoid-chondroid texture in human hearts, even in embryos, is consistent with the analysis of its interpretation. This disparity is associated with the different age evolution from chondroid to osseous material and with the greater power developed by bovid hearts, requiring a more rigid point of support.
The muscular segments that in continuity make up the ventricular chambers must carry out their movements on a point of support, which we have investigated and termed cardiac fulcrum, the same as a skeletal muscle does in a firm insertion.
Its presence is constant in all the hearts studied, both bovid and human, but its structural characteristics are different. This difference in the intimate analysis of the cardiac fulcrum is undoubtedly related to the resistance that it must oppose to the energetic action of the myocardium in hearts of different sizes.
Beyond its mere mention, until our investigations, no function or meaning of its presence had ever been assigned, as well as its lack of description in human beings.
Authors contributions
Trainini Jorge: Project Management; Wernicke Mario: Histology; Beraudo Mario: Anatomy; Trainini Alejandro: Anatomy.
- Torrent Guasp F. Structure and function of the heart. Rev Esp Cardiol.1998; 51: 91-102.
- Buckberg GD, Coghlan HC, Torrent-Guasp F. The structure and function of the helical heart and its buttress wrapping. V. Anatomic and physiologic considerations in the healthy and failing heart. Semin Thorac Cardiovasc Surg. 2001 Oct;13(4):358-85. doi: 10.1053/stcs.2001.29957. PMID: 11807734.
- Trainini JC, Beraudo M, Wernicke M, Carreras-Costa F, Trainini A. Anatomical Investigation of the Cardiac Apex. Rev Argent Cardiol. 2022; 90:118-123. http://dx.doi.org/10.7775/rac.v90.i2.20498
- Trainini JC, Elencwajg B, Herreros J. New Physiological Concept of the Heart. Ann Transplant Res. 2017; 1(1): 1001.
- Trainini JC, Herreros J, Elencwajg B, Trainini A, Lago N. Myocardium dissection. Rev Argent Cardiol. 2017; 85:40-46. http://dx.doi.org/10.7775/rac.v85.i1.10198
- Ballester M, Ferreira A, Carreras F. The myocardial band. Heart Fail Clin. 2008 Jul;4(3):261-72. doi: 10.1016/j.hfc.2008.02.011. PMID: 18598979.
- Torrent-Guasp F, Buckberg GD, Clemente C, Cox JL, Coghlan HC, Gharib M. The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Semin Thorac Cardiovasc Surg. 2001 Oct;13(4):301-19. doi: 10.1053/stcs.2001.29953. PMID: 11807730.
- Trainini J, Valle Cabezas J, Carreras-Costa F, Beraudo, M, Wernicke M. “Cardiac Energy”. Clin Exp Invest. 2022; 3(1): 7-7. Doi:10.31487/j.CEI.2022.01.02
- Pedrizzetti G, Arvidsson PM, Töger J, Borgquist R, Domenichini F, Arheden H, Heiberg E. On estimating intraventricular hemodynamic forces from endocardial dynamics: A comparative study with 4D flow MRI. J Biomech. 2017 Jul 26;60:203-210. doi: 10.1016/j.jbiomech.2017.06.046. Epub 2017 Jul 5. PMID: 28711164.
- Maksuti E, Carlsson M, Arheden H, Kovács SJ, Broomé M, Ugander M. Hydraulic forces contribute to left ventricular diastolic filling. Sci Rep. 2017 Mar 3;7:43505. doi: 10.1038/srep43505. PMID: 28256604; PMCID: PMC5334655.
- Trainini JC, Trainini A, Valle Cabezas J, Cabo J. Left Ventricular Suction in Right Ventricular Dysfunction. EC Cardiology. 2019; 6(6): 572-57.
- Best A, Egerbacher M, Swaine S, Pérez W, Alibhai A, Rutland P, Kubale V, El-Gendy SAA, Alsafy MAM, Baiker K, Sturrock CJ, Rutland CS. Anatomy, histology, development and functions of Ossa cordis: A review. Anat Histol Embryol. 2022 Nov;51(6):683-695. doi: 10.1111/ahe.12861. Epub 2022 Sep 8. PMID: 36073246; PMCID: PMC9826330.
- Moittié S, Baiker K, Strong V, Cousins E, White K, Liptovszky M, Redrobe S, Alibhai A, Sturrock CJ, Rutland CS. Discovery of os cordis in the cardiac skeleton of chimpanzees (Pan troglodytes). Sci Rep. 2020 Jun 10;10(1):9417. doi: 10.1038/s41598-020-66345-7. PMID: 32523027; PMCID: PMC7286900.
- Trainini J, Lowenstein J, Beraudo M, Wernicke M, Trainini A, Llabata VM, Carreras CF. Myocardial torsion and cardiac fulcrum. Morphologie. 2021 Feb;105(348):15-23. doi: 10.1016/j.morpho.2020.06.010. Epub 2020 Jul 6. PMID: 32646845.
- Carlos TJ, Beraudo M, Wernicke M, Alejandro T, Jorge L, Bastarrica ME. Myocardial Torsion and Cardiac Fulcrum. J Morphol Anat. 2021; 5: S1.
- Trainini J, Beraudo M, Wernicke M, Trainini A. Haber Lowenstein D, Bastarrica M, Martino DC, Lowenstein J. The myocardial support. Rev Argent Cardiol. 2021; 89: 217-223. http://dx.doi.org/10.7775/rac.v89.i3.20407
- Trainini JC, Beraudo M, Wernicke M, Carreras-Costa F, Trainini A, M, Mora Llabata V, Cabezas Valle J, Lowenstein Haber D, Bastarrica ME, Lowenstein JA. Evidence that the myocardium is a continuous helical muscle with one insertion. REC: Cardio Clinics. 2022; 57:194-202 https://doi.org/10.1016/j.rccl.2022.01.006
- Sosa Olavarría A, Martí Peña A, Martínez MA, Zambrana Camacho J, Ulloa Virgen J, Zurita Peralta J, Alcedo A, Pérez-Canto GCH, Vázquez E, Yassef Antúnez Montes O, Moncayo R, Belgoff S. Trainini cardiac fulcrum in the fetal heart. Rev Peru Ginecol Obstet. 2023; 69(4). DOI: https://doi.org/10.31403/rpgo.v69i2579
- Henson RE, Song SK, Pastorek JS, Ackerman JJ, Lorenz CH. Left ventricular torsion is equal in mice and humans. Am J Physiol Heart Circ Physiol. 2000 Apr;278(4):H1117-23. doi: 10.1152/ajpheart.2000.278.4.H1117. PMID: 10749705.
- Trainini JC, Elencwajg B, López-Cabanillas N, Herreros J, Lowenstein J. Ventricular torsion and cardiac suction effect: The electrophysiological analysis of the cardiac band muscle. Interventional Cardiol. 2017; 9(1): 45-51.
- Trainini JC, Elencwajg B, López-Cabanillas N, Herreros J, Lago N. Electrophysiological Bases of Torsión and Suction in the Continuous Cardiac Band Model. Anat Physiol. 2015; 5:S4-001 http://dx.doi.org/10.4172/2161-0940. S4-001.
- Trainini JC, Elencwajg B, López-Cabanillas N, Herreros J, Lowenstein J. Ventricular torsion and cardiac suction effect: The electrophysiological analysis of the cardiac band muscle. Interventional Cardiol. 2017; 9 (1): 45-51.
- Poveda F, Gil D, Martí E, Andaluz A, Ballester M. Tractographic study of the helical anatomy of the ventricular myocardium using diffusion tensor magnetic resonance imaging. Rev Esp Cardiol. 2013;66:782-90.
- Carreras F, Ballester M, Pujadas S, Leta R, Pons-Llado G. Morphological and functional evidences of the helical heart from non-invasive cardiac imaging. Eur J Cardiothorac Surg. 2006 Apr;29 Suppl 1:S50-5. doi: 10.1016/j.ejcts.2006.02.061. Epub 2006 Mar 24. PMID: 16563788.
- Torrent Guasp F. Agonist-antagonist mechanics of the descending and ascending segments of the ventricular myocardial band. Rev Esp Cardiol. 2001; 54:1091-102.