More Information
Submitted: December 19, 2023 | Approved: January 08, 2024 | Published: January 09, 2024
How to cite this article: Weisman SM, Angiolillo DJ. Aspirin for Primary Prevention of Cardiovascular Disease: What We Now Know. J Cardiol Cardiovasc Med. 2024; 9: 006-013.
DOI: 10.29328/journal.jccm.1001172
Copyright License: © 2024 Weisman SM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cardiovascular disease; Primary prevention; Aspirin; Patient-centric; Risk assessment
Aspirin for Primary Prevention of Cardiovascular Disease: What We Now Know
Steven M Weisman1* and Dominick J Angiolillo2
1Lumanity, Inc. Morristown, NJ, USA
2Division of Cardiology, Department of Medicine, College of Medicine-Jacksonville, University of Florida, Jacksonville, FL, USA
*Address for Correspondence: Steven M Weisman, PhD, Lumanity, Inc. Morristown, NJ, USA, Email: [email protected]
Cardiovascular disease (CVD), including coronary artery disease and stroke, is the leading cause of death worldwide. Advances in primary and secondary prevention of CVD have improved patient prognoses and outcomes, however, it is imperative that the clinician and patient engage in early risk factor screening and preventive management of modifiable risk factors for CVD. In addition to blood lipid and blood pressure lowering medications, aspirin has been a long-standing therapy targeted to the prevention of CVD based on its antiplatelet and anti-inflammatory activity. However, recent articles and reports on updates to clinical guidelines for the primary prevention of CVD have resulted in confusion about aspirin recommendations. This review aims to assess the latest guidance on aspirin in CVD prevention and how to identify appropriately at-risk patients who may benefit from low-dose aspirin therapy as part of their CVD preventive healthcare choices. Additionally, this review will provide practical application guidance about clinician-patient conversations to clearly explain the benefits and risks of aspirin use and ensure a patient-centric decision to initiate aspirin therapy.
ACC/AHA: American College of Cardiology/American Heart Association; ASCVD: Atherosclerotic Cardiovascular Disease; BP: Blood Pressure; CAC: Coronary Artery Calcium; CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; CI: Confidence Interval; COR: Class of Recommendation; COX: Cyclooxygenase; CVD: Cardiovascular Disease; GI: Gastrointestinal; HDL-C: High-Density Lipoprotein-Cholesterol; hsCRP: high-sensitivity C-Reactive Protein; LDL-C: Low-Density Lipoprotein-Cholesterol; LOE: Level of Certainty; OR: Odds Ratio; PCE: Pooled Cohort Equation; PGI2: Prostacyclin I2; PVD: Peripheral Vascular Disease World Health Organization (WHO); RCT: Randomized Clinical Trials; SBP: Systolic Blood Pressure; TIA: Transient Ischemic Attack; TXA2: Thromboxane A2; USPSTF: US Preventive Services Task Force.
Cardiovascular disease (CVD) represents a major public health challenge, with devastating implications for global morbidity and mortality. According to the World Health Organization (WHO), CVD remains the leading cause of death worldwide, responsible for a staggering 17.9 million fatalities in 2019, which accounted for approximately 32% of all global deaths [1]. The overwhelming majority of these deaths, around 85%, were attributed to heart attacks and strokes, highlighting the urgent need for effective strategies to prevent these life-threatening events. In the United States (US), CVD accounted for 928,741 deaths in the year 2020. Among all CVD deaths, coronary heart disease was the leading cause of mortality (41.2%) followed by stroke (17.3%) and other CVD (16.8%) [2].
Furthermore, the burden of CVD extends beyond absolute mortality. Premature death, often defined as adults less than 65 years of age, due to CVD results in significant potential years of life lost and concomitant loss of economic productivity [3]. Heart disease and stroke are reported to incur $155 billion in lost (job) productivity annually [2]. Given the costs of premature deaths from CVD, effective interventions targeting modifiable risk factors can significantly reduce the incidence of CVD, thereby potentially preventing a considerable number of these premature deaths.
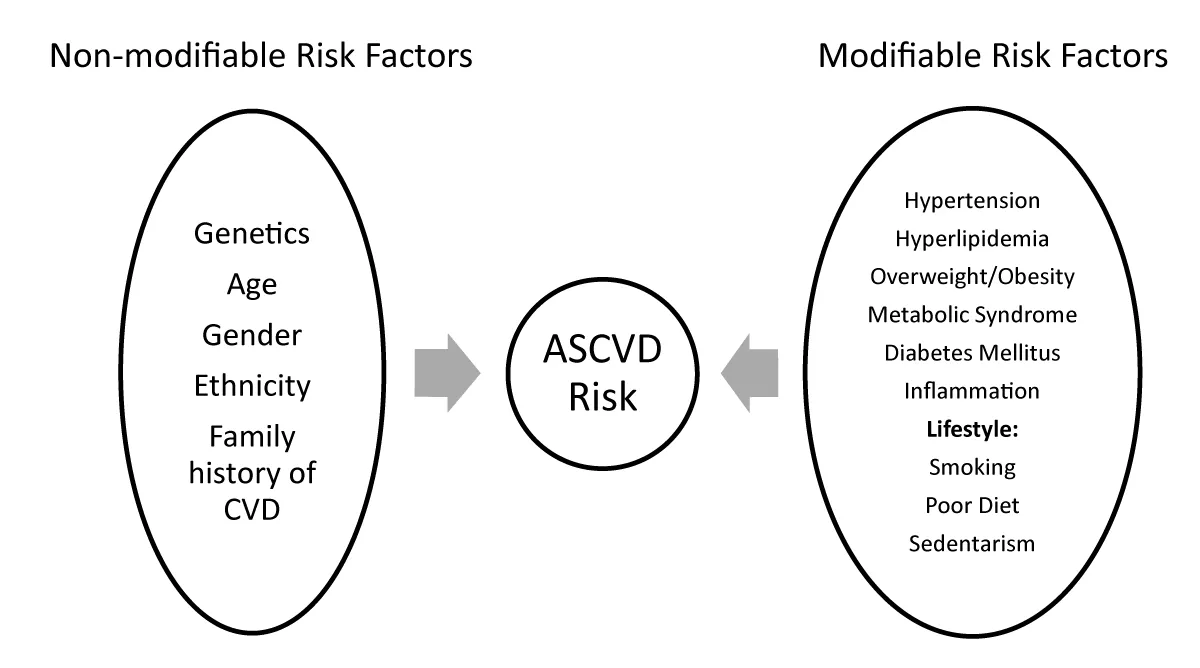
To effectively combat CVD, it is essential to understand the multifactorial nature of its development. A combination of non-modifiable risk factors, such as age and gender, and modifiable risk factors, including overweight and obesity, blood cholesterol levels, blood pressure, type 2 diabetes, inflammation, sedentary lifestyle, unhealthy diet, and tobacco use, contribute to the pathogenesis of CVD (Figure 1, CDC, [4]).
Figure 1: Risk Factors for Atherosclerotic Cardiovascular Disease (ASCVD).
In addition to blood lipid and blood pressure lowering medications, aspirin has been a long-standing therapy targeted to prevent CVD based on its antiplatelet and anti-inflammatory activity [5]. However, recent articles and reports on updates to clinical guidelines for the primary prevention of CVD have resulted in confusion about aspirin recommendations. This review aims to assess the latest guidance on aspirin in CVD prevention and how to identify appropriately at-risk patients who may benefit from low-dose aspirin therapy as part of their preventive healthcare choices.
Primary prevention of CVD
Primary prevention of CVD aims to prevent the onset of disease through strategies to reduce known risk factors for CVD. Risk assessment constitutes a pivotal step in the contemporary approach to primary prevention of atherosclerotic cardiovascular disease (ASCVD). This process traditionally involves the evaluation of a patient’s 10-year risk for ASCVD, aiding in the identification of individuals falling into higher-risk categories. This differentiation is of utmost importance as it empowers healthcare providers to determine the most suitable strategies for mitigating the likelihood of ASCVD while minimizing the number of individuals requiring treatment, particularly in the context of statin and antihypertensive therapies. The ASCVD risk score, an established national guideline developed by the American College of Cardiology, stands as the cornerstone of this assessment procedure. It encompasses a comprehensive calculation of an individual’s 10-year risk of encountering cardiovascular issues such as heart attacks or strokes. This risk evaluation takes into consideration a plethora of variables, including age, gender, ethnicity, cholesterol levels, blood pressure, medication utilization, diabetic status, and smoking habits. By considering these multifaceted factors, healthcare professionals can make well-informed decisions and tailor preventive measures to suit the distinct risk profile of each patient [6]. Table 1 outlines current recommendations for management of modifiable risk factors including blood pressure, cholesterol, and lifestyle choices.
| Table 1: ASCVD Recommendations from ACC/AHA Guidelines. | |
| The 2017 Hypertension Clinical Practice Guidelines [8] highlight multifaceted blood pressure management: | Nonpharmacological interventions encompass weight loss, heart-healthy dietary patterns, sodium reduction, dietary potassium supplementation, structured exercise, and moderate alcohol intake. |
| Individuals with an estimated 10-year ASCVD risk ≥ 10% and average systolic BP ≥ 130 mm Hg or average diastolic BP ≥ 80 mm Hg should consider BP-lowering medications. | |
| BP target of < 130/80 mm Hg is advocated for confirmed hypertension with 10-year ASCVD risk ≥ 10%. | |
| Initiation of BP-lowering medication is recommended in cases of estimated 10-year ASCVD risk < 10%, with systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg. | |
| The 2018 Cholesterol Clinical Practice Guidelines [7] underscore personalized management approaches: | Intermediate ASCVD-risk individuals (≥ 7.5% to < 20% 10-year risk) are advised statin therapy, with a preference for moderate-intensity statins following risk discussion. |
| Focus on achieving LDL-C reductions of ≥ 30% in intermediate-risk patients, escalating to ≥ 50% for high-risk individuals (≥ 20% 10-year risk). | |
| Adults aged 40 to 75 with diabetes warrant moderate-intensity statin therapy, irrespective of estimated ASCVD risk. | |
| Maximal statin therapy is recommended for those aged 20 to 75 with LDL-C levels ≥ 190 mg/dL. | |
| Lifestyle Adjustments and the 2019 ACC/AHA Guideline [9]: Diet and Nutrition, and Physical Activity |
Adopting a diet abundant in vegetables, fruits, legumes, nuts, whole grains, and fish, strategically lowers the risk of atherosclerotic cardiovascular disease (ASCVD). |
| Substitution of saturated fats with monounsaturated and polyunsaturated fats as a tactical measure to curtail ASCVD risk. Recommending the management of cholesterol and sodium intake serves as a cornerstone in mitigating ASCVD risk |
|
| Restriction on the consumption of processed meats, refined carbohydrates, and sweetened beverages. | |
| A target of a minimum of 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic activity per week is suggested, in a concerted effort to reduce ASCVD risk. | |
Low-dose aspirin for CVD risk reduction
The history of aspirin is a captivating tale that revolves around its discovery and its wide array of applications. The origins of aspirin can be traced back to salicylates found in the bark of willow trees, with their pain-relieving properties documented for centuries, spanning back to the Sumerians and the practices of Hippocrates [10]. Notably, in the 18th century, Reverend Edward Stone conducted a clinical trial utilizing willow bark powder to address fever—a pioneering instance of a clinical trial.
Advancements continued into the 19th century as the active compound in willow, salicin, was successfully isolated and investigated for its efficacy in managing acute rheumatism. Throughout its evolution, aspirin’s applications expanded significantly. Beyond its role in pain relief and fever reduction, it found utility in addressing cardiovascular risk, angina pectoris, myocardial infarction, ischemic stroke, and even rheumatoid arthritis.
Aspirin’s mechanism of action revolves around the irreversible blockade of cyclooxygenase (COX) enzymes, specifically COX-1 and COX-2. This inhibition is achieved through the acetylation of specific serine residues within these enzymes. Consequently, the normal enzymatic activity is disrupted. This process plays a pivotal role in impeding the access of arachidonic acid to the COX catalytic site, leading to the suppression of thromboxane A2 (TXA2) and prostacyclin I2 (PGI2) production [11].
Aspirin functions as an antiplatelet agent by permanently inhibiting the enzyme cyclooxygenase-1 (COX-1) within platelets. COX-1 is vital for producing thromboxane A2, a potent activator of platelets derived from arachidonic acid [11]. By inhibiting the enzyme cyclooxygenase, aspirin interferes with the production of prostaglandins, thereby reducing platelet activation and aggregation, which is crucial in preventing blood clots within arteries. This antiplatelet effect is particularly important in the context of cardiovascular diseases, including atherosclerosis and coronary artery disease, as it lowers the risk of heart attacks and strokes. Additionally, aspirin also hinders COX-2, contributing to its anti-inflammatory effects. Alongside this, other mechanisms are suggested, including decreased prostaglandin production and interactions with immune cells and cytokines.
Aspirin’s mechanism of action yields cardiovascular benefits by reducing the risk of CVD events. However, it introduces a well-documented conundrum-an increased susceptibility to bleeding events, which span a spectrum of clinical significance. Among these, gastrointestinal (GI) bleeding emerges as the most frequently encountered consequence of long-term aspirin use [12]. Aspirin use is associated with an elevated risk of GI bleeding, including the development of stomach ulcers and bleeding in the small intestine. This heightened risk is attributed to aspirin’s inhibition of prostaglandin production, which disrupts the protective mechanisms of the GI tract, rendering it more susceptible to damage from stomach acid. The risk of GI bleeding is further compounded with chronic or high-dose aspirin use [13]. GI bleeding triggered by aspirin can lead to serious complications, including chronic anemia due to ongoing blood loss, and in some instances, life-threatening events such as perforated ulcers or severe gastrointestinal hemorrhage [14].
Current guidelines on the use of low-dose aspirin in CVD prevention
In recent years, guidelines regarding the recommendations for low-dose aspirin use in the primary prevention of CVD have been issued by the ACC/AHA Task Force on Clinical Practice Guidelines [9] and the US Preventive Services Task Force [15]. Both guidelines are based on the Aspirin in Reducing Events in the Elderly trial [16] and several meta-analyses [17-19]. Below is a summary of each report’s findings and recommendations and Table 2 summarizes the recommendations of each task force.
| Table 2: Current Low-Dose Aspirin Use Recommendations. | |||||
| Age Range (years) | Risk Level | Recommendation | COR**/ Suggestion for Practice Grade* | LOE*/Level of Certainty (net benefit)** |
|
| 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease** | 40-70 | “Elevated risk” per PCE or based on the presence of specific ASCVD risk factors |
Low-dose aspirin (75-100 mg orally daily) might be considered for the primary prevention of ASCVD among select adults 40 to 70 years of age who are at higher ASCVD risk but not at increased bleeding risk. | IIb** | A** |
| > 70 | NA | Low-dose aspirin (75-100 mg orally daily) should not be administered on a routine basis for the primary prevention of ASCVD among adults > 70 years of age | III: Harm** | B-R** | |
| NA | NA | Low-dose aspirin (75-100 mg orally daily) should not be administered for the primary prevention of ASCVD among adults of any age who are at increased risk of bleeding. | III: Harm** | C-LD** | |
| 2022 Aspirin Use to Prevent Cardiovascular Disease - US Preventive Services Task Force Recommendation Statement* | 40-59 | ≥ 10% 10-year CVD Risk |
The decision to initiate low-dose aspirin use for the primary prevention of CVD in adults aged 40 to 59 years who have a 10% or greater 10-year CVD risk should be an individual one. Evidence indicates that the net benefit of aspirin use in this group is small. Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit. | C* | Moderate* |
| ≥ 60 | NA | Recommends against initiating low-dose aspirin use for the primary prevention of CVD in adults 60 years or older. |
D* | Moderate* | |
| *Evidence rating system for USPSTF (Grade Definitions. U.S. Preventive Services Task Force. October 2018). ** Evidence rating system for ACC/AHA (https://cpr.heart.org/en/resuscitation-science/cpr-and-ecc-guidelines/tables/applying-class-of-recommendation-and-level-of-evidence). PCE = Pooled Cohort Equation. COR = Class of Recommendation; LOE = Level of Certainty; COR IIb/LOE A = high-quality evidence showing treatment may be reasonable, but effectiveness is not well established; COR III/LOE B-R = moderate-quality evidence showed no benefit and potential harm; COR III/LOE C-LD = week/limited data. | |||||
In 2022, the USPSTF issued a new Recommendation Statement on the use of Aspirin in CVD prevention to update and replace the 2016 recommendations [15]. A systematic review was conducted to evaluate the efficacy of aspirin in reducing the risk of CVD events (MI and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD as well as the harms (bleeding) associated with aspirin use.
The systematic review included 13 randomized clinical trials (RCT) involving 161,680 participants. The mean age ranged from 53 to 74 years of age and included 22,000 participants younger than 50 years and more than 37,000 participants 70 years or older. Results showed that aspirin use (≤ 100 mg/d) was associated with decreased risk of MI and stroke but not cardiovascular mortality or all-cause mortality. Pooled analyses showed that low-dose aspirin use lowered the risk of non-fatal MI and nonfatal ischemic stroke and that the absolute magnitude of benefit was greater in individuals with higher CVD risk.
A similar approach was employed to evaluate the risk of bleeding with low-dose aspirin use. Pooled analyses with low-dose aspirin (≤ 100 mg/d) showed a 58% (OR, 1.58 [95% CI, 1.38-1.80]) associated increase in major GI bleeding and a 31% (OR, 1.31 [95% CI, 1.11-1.54]) increase in the risk of intracranial bleeds. However, low-dose aspirin use was not associated with fatal hemorrhagic stroke. Of note, though the relative risk of bleeding does not differ by age, the absolute incidence of bleeding was shown to increase with age, especially after 60 years of age [15].
A microsimulation modeling study was conducted to estimate the net benefit of low-dose aspirin use [20,21]. The simulation estimated that low-dose aspirin use provided the greatest, albeit modest, benefits in men and women aged 40-59 years with 10% or greater 10-year CVD risk. Furthermore, the data showed that initiating aspirin use in persons aged 60-69 had little effect with a slightly negative effect of life-years gained. Initiation of aspirin use in those aged 70-79 years had a negative effect on both quality-adjusted life-years and life-years regardless of CVD risk level. Based on these findings, the USPSTF concluded that aspirin use has a small net benefit for individuals between 40-59 years of age with a 10% or greater 10-year CVD risk and that initiation of aspirin use beyond this age range yields no benefits in the prevention of CVD.
As the USPSTF 2022 Recommendation Statement replaces the 2016 Recommendation Statement it is important to note the key changes in guidance for the use of low-dose aspirin in CVD prevention. The 2022 USTSTF report has changed both the age ranges and recommendation grades for aspirin use in the prevention of CVD. The latest recommendation has expanded the age range for initiating low-dose aspirin therapy from 50-59 to 40-59 years of age with a 10% or greater 10-year CVD risk and specifies that the decision to initiate aspirin therapy should be an individual one. Initiation of aspirin therapy in adults between 60-69 years of age was previously based on individual choice for patients who place a higher value on the potential benefits versus the potential harms, however, the 2022 USPSTF guidance does not recommend initiating low-dose aspirin therapy over the age of 60 and to discontinue aspirin therapy after the age of 75 years. The previous recommendation did not address adults younger than 50 or older than 70 years of age due to insufficient evidence.
The 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease [9] also addressed aspirin use in primary prevention of CVD. The report concluded that the estimated ASCVD risk tends to exceed the actual risk observed during study follow-up. Collectively, with evidence that observed bleeding risk is less well correlated with baseline estimated ASCVD risk, the 2019 Guideline lowered the class of recommendation (COR) for prophylactic aspirin use to IIb which indicates the treatment usefulness and efficacy is less well established by the evidence. The evidence also supported the removal of a specific pooled cohort equation (PCE) threshold, which was previously set at greater than ≥ 10% 10-year CVD risk, and now indicates individuals “at higher ASCVD risk but not at increased bleeding risk.”
Based on the COR and the level of evidence (A – high-quality evidence) the use of aspirin in primary prevention should be determined on a case-by-case basis for patients without signs or symptoms of CVD between 40-70 years of age to determine if the benefits outweigh the risks and should include other risk-enhancing factors to tailor preventive health decisions. More specifically, the best-suited candidate to benefit from low-dose aspirin (75-100 mg/d) use would be an individual at higher ASCVD risk who does not achieve optimal control of other risk-enhancing factors. For individuals over the age of 70 years, the Guideline states that prophylactic aspirin use should not be administered. Additionally, individuals of any age with an increased risk for bleeding should not use aspirin in the primary prevention of CVD.
Appropriately at-risk patient profile
Based on evidence to date and recent clinical guidelines, the appropriately at-risk patient for low-dose aspirin therapy may be an individual between 40-70 years of age, without preexisting diagnoses or symptoms of CVD but with a higher risk for ASCVD, who is at lower risk for bleeding and who seeks to be proactive about CVD prevention and places higher value on the potential benefit of taking low-dose aspirin than the risk for bleeding (Table 3). The clinician should use discretion in determining risk as either ≥ 10% 10-year CVD Risk and/or consideration of risk-enhancing factors. The targeted aspirin dose should be between 75-100 mg/d with most over-the-counter options available at 81 mg/dose.
| Table 3: Criteria Assessment for the appropriately at-risk patient who may benefit from low-dose aspirin therapy. | |
| Appropriate “at-risk” individual for low-dose aspirin in CVD Prevention | |
| Age range | 40 – 59 years of age |
| 60 – 70 years of age with low risk for bleeding and falls/accidents | |
| ASCVD Risk | ≥ 10% 10-year CVD Risk - OR- |
| ≥ 7.5%* 10-year CVD risk with consideration of risk-enhancing factors and social determinants of health | |
| Bleeding Risk | Low |
| Willing to take aspirin | Yes |
| Understand the Benefits and Risks of taking Aspirin | Yes; based on a discussion with a clinician |
| * 2019 ACC/AHA “intermediate risk” ≥ 7.5% - 20% 10-year CVD Risk | |
Clinical application
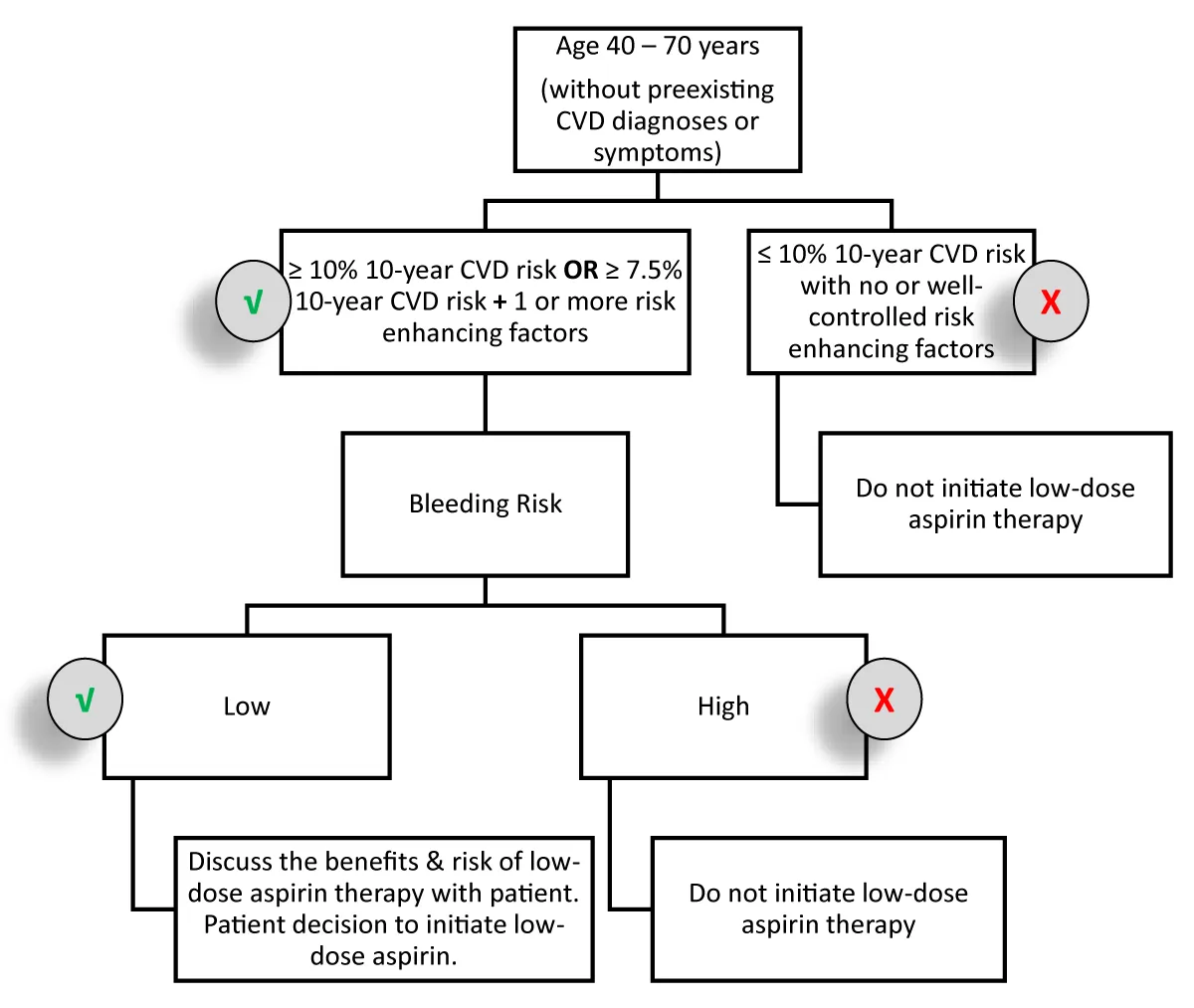
Low-dose aspirin use may provide modest benefits to appropriately at-risk patients [9,15]. Clinicians should assess patient risk for CVD and initiate conversations with appropriate at-risk patients about including low-dose aspirin use in their preventative healthcare strategies (Figure 2). The risk assessment should include the use of validated tools to assess CVD and bleeding risks as well as risk-enhancing factors. With these tools and considerations clinicians may then use their knowledge and experiential discretion to determine if a patient may benefit from low-dose aspirin therapy.
Figure 2: Risk Evaluation Cascade for Assessment Low-dose Aspirin Initiation.
Assessing ASCVD risk
The ACC/AHA Guidelines endorse the use of the US-derived PCE (Table 4) as a valuable tool for estimating the 10-year risk of severe ASCVD events, including coronary death, nonfatal myocardial infarction, and fatal or nonfatal stroke [6]. What sets the PCE apart is its meticulous customization, accounting for variations in sex and race, with specific adjustments for both white and black populations. Notably, the inclusion of stroke as an endpoint enhances its utility, particularly in identifying modifiable risks among women and individuals of African descent.
| Table 4: Risk Assessment Tools for ASCVD. | |||
| Risk Assessment Tool | ACC ASCVD Risk Estimator Plus | Framingham General CVD Risk Profile |
Reynolds Risk Score |
| Variables | Age Sex Race Total cholesterol HDL-C SBP Antihypertensive therapy History of diabetes mellitus Current smoking |
Age Sex Total cholesterol HDL-C SBP Antihypertensive therapy History of diabetes mellitus Current smoking |
Age Sex Total cholesterol HDL-C SBP Current smoking hsCRP level Parental history of MI before age 60 yr |
| Outcomes | Estimating 10-year absolute rates of atherosclerotic cardiovascular disease (ASCVD) events | Estimates 10-year risk of CVD (CAD, Stroke, PVD, CHF, cardiac death, TIA) |
Estimates risk of having a future heart attack, stroke, or other major heart disease in the next 10 years. |
| Resource | Online: https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/ |
Online: https://reference.medscape.com/calculator/252/framingham-risk-score-2008 |
Online: http://www.reynoldsriskscore.org/ |
Recent research confirms the effective calibration of the PCE, especially around critical decision thresholds, such as the 7.5% to 10% 10-year ASCVD risk, within the broader US population [6]. However, it is essential for healthcare professionals to exercise caution and consider specific contextual factors when interpreting PCE results. Instances may arise where the PCE could underestimate risk, especially in individuals with lower socioeconomic status or those managing chronic inflammatory conditions. Conversely, it might overestimate risk in populations with a projected 10-year risk exceeding 10%, individuals with higher socioeconomic status, or those consistently receiving screening and preventive care.
Comparatively, the Framingham General CVD Risk Profile offers a similar level of utility as the PCE but encompasses a broader range of outcomes, including ASCVD events, unstable angina/coronary insufficiency, transient ischemic attack, claudication, and heart failure, which may not be directly preventable with lipid-lowering therapy [6]. On the other hand, the Reynolds Risk Score shows slightly improved performance over the PCE in specific higher socioeconomic and lower-risk populations and includes coronary revascularization as an endpoint. However, it is worth noting that certain aspects of the Framingham and Reynolds risk assessment tools, such as their suitability for nonwhite patients, calibration to the hard ASCVD endpoint, and reclassification by coronary artery calcium (CAC) scoring, may limit their alignment with ACC/AHA clinical practice guidelines.
It is crucial to emphasize that the ACC/AHA clinical practice guidelines stress that while these risk assessment tools serve as valuable initial assessments, they should not be the sole determinants when making decisions related to the primary prevention of ASCVD. Healthcare professionals should consider a holistic approach, considering patient-specific factors, and clinical judgment when developing prevention strategies.
In addition to utilizing risk assessment tools, clinicians should consider patient-specific factors to formulate a comprehensive prevention strategy. Risk-enhancing factors for atherosclerotic cardiovascular disease (ASCVD) encompass a diverse array of conditions and traits that elevate the probability of cardiovascular events. These encompass a family history of premature ASCVD, primary hypercholesterolemia characterized by elevated LDL-C or non-HDL-C levels, the presence of metabolic syndrome, chronic kidney disease marked by decreased eGFR, chronic inflammatory conditions such as psoriasis or rheumatoid arthritis, a history of premature menopause or pregnancy-related conditions like preeclampsia, individuals of high-risk race/ethnicity, and specific lipid and biomarker measurements, such as elevated triglycerides, high-sensitivity C-reactive protein (hsCRP), lipoprotein(a), and apolipoprotein B levels. Recognizing these risk-enhancing factors is pivotal in evaluating an individual’s comprehensive ASCVD risk profile and guiding the implementation of preventive measures. In addition to the established risk-enhancing factors, coronary artery calcium (CAC) scoring may prove useful in establishing an individual’s risk. A CAC score measures the amount of calcified plaque in the coronary artery and is predictive of the likelihood of a future heart attack or stroke. PCEs are more reliable when an individual has three or more risk enhancers. When risk decision remains uncertain for intermediate‐risk patients with < 3 risk enhancers, CAC evaluation provides reliable insights into patients’ risks [21,22]. Table 5 illustrates the 2019 ACC/AHA Risk Enhancing Factors below for reference.
| Table 5: 2019 ACC/AHA Risk-Enhancing Factors for ASCVD. |
| Risk-Enhancing Factors |
| Family history of premature ASCVD (males, age < 55 y; females, age < 65 y) |
| Primary hypercholesterolemia (LDL-C, 160–189 mg/dL [4.1–4.8 mmol/L]; non–HDL-C 190–219 mg/dL [4.9–5.6 mmol/L])* |
| Metabolic syndrome (increased waist circumference [by ethnically appropriate cut-points], elevated triglycerides [> 150 mg/dL, nonfasting], elevated blood pressure, elevated glucose, and low HDL-C [< 40 mg/dL in men; < 50 mg/dL in women] are factors; a tally of 3 makes the diagnosis) |
| Chronic kidney disease (eGFR 15–59 mL/min/1.73 m2 with or without albuminuria; not treated with dialysis or kidney transplantation) |
| Chronic inflammatory conditions, such as psoriasis, RA, lupus, or HIV/AIDS |
| History of premature menopause (before age 40 y) and history of pregnancy-associated conditions that increase later ASCVD risk, such as preeclampsia |
| High-risk race/ethnicity (e.g., South Asian ancestry) |
| Lipids/biomarkers: associated with increased ASCVD risk |
| Persistently elevated* primary hypertriglyceridemia (≥ 175 mg/dL, nonfasting) |
| If measured: |
| Elevated high-sensitivity C-reactive protein (≥ 2.0 mg/L) |
| Elevated Lp(a): A relative indication for its measurement is a family history of premature ASCVD. An Lp(a) ≥ 50 mg/dL or ≥ 125 nmol/L constitutes a risk-enhancing factor, especially at higher levels of Lp(a). |
| Elevated apoB (≥ 130 mg/dL): A relative indication for its measurement would be triglyceride ≥ 200 mg/dL. A level ≥ 130 mg/dL corresponds to an LDL-C > 160 mg/dL and constitutes a risk-enhancing factor |
| ABI (< 0.9) |
| *Optimally, 3 determinations for primary hypercholesterolemia ABI indicates ankle-brachial index; AIDS, acquired immunodeficiency syndrome; apoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); and RA, rheumatoid arthritis. Reproduced with permission from Grundy et al. S2.2-4 Copyright © 2018, American Heart Association, Inc., and American College of Cardiology Foundation. |
In addition to CVD risk, healthcare providers must pay particular attention to evaluating bleeding risk in their patients. This consideration becomes especially crucial when dealing with anti-platelet medications such as aspirin, as bleeding stands out as one of the most prevalent side effects associated with their use. Clinicians should also exercise vigilance by considering a patient’s medical history and their existing medication regimen, both of which can significantly elevate the risk of bleeding complications. Table 6 presents a few scenarios known to be associated with an increased risk of bleeding.
| Table 6: 2019 ACC/AHA Factors for Bleeding Risk. |
| Factors Increasing Bleeding Risk: |
| History of prior gastrointestinal bleeding, peptic ulcer disease, or bleeding episodes from other sources |
| Patients aged over 70 years or fall risk |
| Thrombocytopenia |
| Chronic kidney disease (CKD) |
| Concurrent usage of other medications, including NSAIDs, steroids, anticoagulants, and additional antiplatelet agents |
Recent advancements in the field of primary CVD prevention have identified the potential benefits of low-dose aspirin in appropriately selected individuals, a consensus ratified by authoritative organizations such as the American College of Cardiology and American Heart Association (ACC/AHA), along with the U.S. Preventive Services Task Force (USPSTF). In clinical application, healthcare practitioners must assume a pivotal role in orchestrating personalized, nuanced, and comprehensive risk assessments, encompassing a meticulous evaluation of both CVD and bleeding risks to determine the patients who may benefit from aspirin therapy. Moreover, the clinician should conduct meaningful conversations with these patients to clearly explain the benefits and risks of aspirin use and ensure a patient-centric decision to initiate aspirin therapy. Ongoing monitoring of the patient’s CVD and bleeding risks, as well as changes to medications, should be conducted throughout the patient’s care to optimize preventive care.
- World Health Organization. (2021, June 11). Cardiovascular Diseases (CVDs) Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023 Feb 21;147(8):e93-e621. doi: 10.1161/CIR.0000000000001123. Epub 2023 Jan 25. Erratum in: Circulation. 2023 Feb 21;147(8):e622. Erratum in: Circulation. 2023 Jul 25;148(4):e4. PMID: 36695182.
- Carter HE, Schofield D, Shrestha R. Productivity costs of cardiovascular disease mortality across disease types and socioeconomic groups. Open Heart. 2019 Feb 16;6(1):e000939. doi: 10.1136/openhrt-2018-000939. PMID: 30997129; PMCID: PMC6443138.
- CDC – National Center for Chronic Disease Prevention and Health Promotion, Division for Heart Disease and Stroke Prevention – Heart Disease Facts. (2023) https://www.cdc.gov/heartdisease/facts.htm (Accessed: 23 August 2023).
- Lafeber M, Grobbee DE, Spiering W, van der Graaf Y, Bots ML, Visseren FL; SMART Study Group. The combined use of aspirin, a statin, and blood pressure-lowering agents (polypill components) in clinical practice in patients with vascular diseases or type 2 diabetes mellitus. Eur J Prev Cardiol. 2013 Oct;20(5):771-8. doi: 10.1177/2047487312449587. Epub 2012 May 30. PMID: 22649123.
- Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr, Sperling LS, Virani SS, Blumenthal RS. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019 Jun 25;73(24):3153-3167. doi: 10.1016/j.jacc.2018.11.005. Epub 2018 Nov 10. Erratum in: J Am Coll Cardiol. 2019 Jun 25;73(24):3234. PMID: 30423392.
- Grundy SM, Stone NJ; Guideline Writing Committee for the 2018 Cholesterol Guidelines. 2018 Cholesterol Clinical Practice Guidelines: Synopsis of the 2018 American Heart Association/American College of Cardiology/Multisociety Cholesterol Guideline. Ann Intern Med. 2019 Jun 4;170(11):779-783. doi: 10.7326/M19-0365. Epub 2019 May 28. PMID: 31132793.
- Whelton PK, Carey RM. The 2017 Clinical Practice Guideline for High Blood Pressure. JAMA. 2017 Dec 5;318(21):2073-2074. doi: 10.1001/jama.2017.18209. PMID: 29159375.
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Sep 10;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678. Epub 2019 Mar 17. Erratum in: Circulation. 2019 Sep 10;140(11):e649-e650. Erratum in: Circulation. 2020 Jan 28;141(4):e60. Erratum in: Circulation. 2020 Apr 21;141(16):e774. PMID: 30879355; PMCID: PMC7734661.
- Arif H, Aggarwal S. Salicylic Acid (Aspirin) [Updated 2023 Jul 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK519032/
- Capodanno D, Angiolillo DJ. Aspirin for Primary Cardiovascular Risk Prevention and Beyond in Diabetes Mellitus. Circulation. 2016 Nov 15;134(20):1579-1594. doi: 10.1161/CIRCULATIONAHA.116.023164. Epub 2016 Oct 11. PMID: 27729421.
- Weisman SM, Brunton S. Primary Prevention of CVD with Aspirin: Benefits vs Risks. J Fam Pract. 2021 Jul;70(6S):S41-S46. doi: 10.12788/jfp.0222. PMID: 34432623.
- Tai FWD, McAlindon ME. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin Med (Lond). 2021 Mar;21(2):131-134. doi: 10.7861/clinmed.2021-0039. PMID: 33762373; PMCID: PMC8002800.
- Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017 Aug 5;390(10094):613-624. doi: 10.1016/S0140-6736(16)32404-7. Epub 2017 Feb 25. PMID: 28242110.
- US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, Cabana M, Chelmow D, Coker TR, Davis EM, Donahue KE, Jaén CR, Krist AH, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Tseng CW, Wong JB. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022 Apr 26;327(16):1577-1584. doi: 10.1001/jama.2022.4983. PMID: 35471505.
- McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, Shah RC, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Johnston CI, Ryan J, Radziszewska B, Jelinek M, Malik M, Eaton CB, Brauer D, Cloud G, Wood EM, Mahady SE, Satterfield S, Grimm R, Murray AM; ASPREE Investigator Group. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018 Oct 18;379(16):1509-1518. doi: 10.1056/NEJMoa1805819. Epub 2018 Sep 16. PMID: 30221597; PMCID: PMC6289056.
- Zheng SL, Roddick AJ. Association of Aspirin Use for Primary Prevention With Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-analysis. JAMA. 2019 Jan 22;321(3):277-287. doi: 10.1001/jama.2018.20578. Erratum in: JAMA. 2019 Jun 11;321(22):2245. PMID: 30667501; PMCID: PMC6439678.
- Guirguis-Blake JM, Evans CV, Senger CA, O'Connor EA, Whitlock EP. Aspirin for the Primary Prevention of Cardiovascular Events: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016 Jun 21;164(12):804-13. doi: 10.7326/M15-2113. Epub 2016 Apr 12. PMID: 27064410.
- Wang M, Yu H, Li Z, Gong D, Liu X. Benefits and Risks Associated with Low-Dose Aspirin Use for the Primary Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Control Trials and Trial Sequential Analysis. Am J Cardiovasc Drugs. 2022 Nov;22(6):657-675. doi: 10.1007/s40256-022-00537-6. Epub 2022 May 16. PMID: 35570250.
- Dehmer SP, O’Keefe LR, Grossman ES. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: An Updated Decision Analysis for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2022 Apr. (Technical Report, No. 211s.) https://www.ncbi.nlm.nih.gov/books/NBK580862/
- Dehmer SP, O'Keefe LR, Evans CV, Guirguis-Blake JM, Perdue LA, Maciosek MV. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2022 Apr 26;327(16):1598-1607. doi: 10.1001/jama.2022.3385. PMID: 35471506.
- Akintoye E, Afonso L, Bengaluru Jayanna M, Bao W, Briasoulis A, Robinson J. Prognostic Utility of Risk Enhancers and Coronary Artery Calcium Score Recommended in the 2018 ACC/AHA Multisociety Cholesterol Treatment Guidelines Over the Pooled Cohort Equation: Insights From 3 Large Prospective Cohorts. J Am Heart Assoc. 2021 Jun 15;10(12):e019589. doi: 10.1161/JAHA.120.019589. Epub 2021 Jun 7. PMID: 34092110; PMCID: PMC8477885.