More Information
Submitted: January 03, 2024 | Approved: January 17, 2024 | Published: January 18, 2024
How to cite this article: Jogimahanti AV, Honan KA, Ahmed T, Leon-Novelo L, Khair T. The Effect of SGLT-2i and GLP-1RA on Major Cardiovascular Conditions: A Meta-Analysis. J Cardiol Cardiovasc Med. 2024; 9: 014-025.
DOI: 10.29328/journal.jccm.1001173
Copyright License: © 2024 Jogimahanti AV, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Heart failure; Glucagon-like peptide 1; Sodium-glucose co-transporter 2; Myocardial infarction; Type 2 Diabetes; Cardiovascular death
Abbreviations: SGLT-2 Inhibitors: Sodium-glucose co-transporter 2 inhibitors; GLP-1RA Agonists: Glucagon-like Peptide-1 agonists; FDA: Food and Drug Administration; T2DM: Type 2 Diabetes Mellitus; AF: Atrial Fibrillation; MI: Myocardial Infarction; HF: Heart Failure
The Effect of SGLT-2i and GLP-1RA on Major Cardiovascular Conditions: A Meta-Analysis
Arjun V Jogimahanti1*, Kevin A Honan1, Talha Ahmed2, Luis Leon-Novelo3 and Tarif Khair2
1University of Texas Medical School at Houston, Department of Internal Medicine, Houston, TX, United States of America
2University of Texas Medical School at Houston, Department of Cardiology, Houston, TX, United States of America
3University of Texas School of Public Health, Department of Biostatistics, Houston, TX, United States of America
*Address for Correspondence: Arjun V Jogimahanti, M.D., University of Texas Medical School at Houston, 6431 Fannin Street, Houston, TX 77030, Email: arjun.v.jogimahanti@uth.tmc.edu
Purpose: Sodium-glucose co-transporter 2 inhibitors (SGLT-2i) and Glucagon-like Peptide-1 Receptor Agonists (GLP-1RA) are two common anti-hyperglycemic agents prescribed by clinicians. The effects on cardiovascular conditions such as Heart Failure (HF) hospitalization, stroke, Myocardial Infarctions (MI), and other cardiovascular conditions are not well studied. The purpose of this study is to analyze existing data on the effect of SGLT-2i and GLP-1RA on preventing these cardiovascular conditions.
Methods: A retrospective meta-analysis of all existing literature on the effect of SGLT-2i and GLP-1RA on the rates of the following categories were researched: primary cardiovascular outcomes, HF hospitalizations, Atrial Fibrillation (AF), stroke, MI, and HF symptoms. Hazard ratios for each category were obtained, and the overall Hazard Ratio (HR) to determine overall statistical significance was computed.
Result: Both medication classes provided a statistically significant reduction in preventing major cardiovascular events. Only SGLT-2i provided a statistically significant reduction in heart failure hospitalizations. Only GLP-1RA provided a statistically significant reduction in preventing stroke. Neither medication class provided a statistically significant benefit in preventing myocardial infarctions. More studies are needed on the effects of either SGLT-2i or GLP-1RA agonists on preventing HF symptoms or AF.
Conclusion: This study demonstrates that SGLT-2i and GLP-1RA are important for improving both diabetic and cardiovascular health. In patients at risk for a major cardiovascular event, SGLT-2i or GLP-1RA may be prescribed by clinicians. More studies must be performed to draw more conclusions.
Cardiovascular Disease (CVD) remains the leading cause of morbidity and mortality in patients with diabetes mellitus. Sodium-glucose co-transporter 2 inhibitors (SGLT-2i) and Glucagon-like Peptide-1 Receptor Agonists (GLP-1RA) are two anti-hyperglycemic agents that have emerged as new therapies for patients with Type Two Diabetes Mellitus (T2DM) and are currently being prescribed by a vast number of clinicians. SGLT-2i prevents the reuptake of sodium, glucose, and water at the proximal convoluted tubule of the nephron [1]. GLP-1RA helps stimulate the endogenous production of insulin [2]. While elevated blood glucose and T2DM were the original indications of use for these medications, utility in Heart Failure (HF) and other cardiac conditions have been recently explored. Some of the mechanisms that have been postulated that have helped include afterload reduction as well as improved glycemic control. Afterload reduction, achieved through proper blood pressure management, is thought to improve cardiac strain. Glycemic control is thought to reduce the risk of metabolic events and coronary artery disease [1,2].
Due to initial landmark trials demonstrating the benefit of SGLT-2i, the Food and Drug Administration (FDA) approved the use of this medication class for use in heart failure and other heart-related medical conditions in 2020 [3]. Many other anti-hyperglycemic medications, including GLP-1RA, have been studied for their role in improving cardiovascular outcomes. Some GLP-1RA, including Dulaglutide, have also been approved for the reduction of cardiovascular events [4]. Randomized Controlled Trials (RCTs) have demonstrated the benefit in many aspects of the cardiovascular profile including Major Adverse Cardiac Events (MACE), Heart Failure (HF) hospitalization rates, Atrial Fibrillation (AF) rates, and the development of HF symptoms. Data regarding the benefit of different medications in either of the two medication classes in the aforementioned disease categories needs to be explored further. Thus, the purpose of this meta-analysis is to determine which cardiac diseases have been shown by the current literature to be most positively impacted by SGLT-2i and GLP-1RA and to discuss the relative performance of both drugs in various cardiovascular disease categories. The results of this study would allow clinicians to effectively manage patients with these co-morbidities or at risk for these co-morbidities. If these medications demonstrated statistically significant benefits in any of the studied cardiovascular conditions, this could help reduce the rate of development of the condition or the incidence of severe outcomes.
A literature search, conducted by all authors listed in the authorship, using the databases PubMed and Google Scholar was performed, ranging from the years 2010-2022. The search for studies lasted three months, occurring mostly in the first quarter of 2022. The following keywords and their MeSH terms were used for the search: ‘(sodium‐glucose co‐transporter inhibitor OR SGLT2 inhibitor OR SGLT‐2 inhibitor OR SGLT 2 inhibitor), (Glucagon-like Peptide-1 Receptor agonist OR GLP-1RA OR GLP1 agonist), (heart failure or cardiac failure OR CHF)’. The effect of both drug classes (SGLT-2i and GLP-1RA) on the rates of the following categories was researched: Major Adverse Cardiac Events (MACE), HF symptoms and hospitalizations, AF, stroke, and myocardial infarction. MACE was defined as the traditional three-point definition: death from cardiovascular cause, nonfatal stroke, or nonfatal myocardial infarction. The criteria used for the selection of the categories included the availability of data from chart review and results of prior available studies. While most other studies focus on MACE and HF, other aspects of cardiovascular disease, such as atrial fibrillation, stroke, and MI, are not well-covered. This was decided after a database review on PubMed and Google Scholar platforms. As a result, the decision to study the effects of SGLT-2i and GLP-1RA on these other cardiovascular diseases was made.
Regarding the criteria of studies chosen, those that met the criteria above were included in the study. It is approximated that more than 50% of the search results did not meet the inclusion criteria.
Inclusion criteria and outcomes of interest
Inclusion criteria for the studies included: 1. Large Randomized Controlled Trials (RCTs) or meta-analyses of previously performed RCTs. Large Randomized controlled trials were defined as studies whose patient populations were greater than 1,000 participants. 2. Patients who took either SGLT-2i or GLP-1RA. The outcomes of interest included the Hazard Ratios (HR) and percent changes compared to placebo within each disease type for both SGLT-2i and GLP-1RA. 3. Studies that assessed the effect of SGLT-2 or GLP-1 agonists on any of the following cardiovascular profiles: primary cardiovascular outcomes, HF symptoms and hospitalizations, AF, stroke, and myocardial infarction.
Statistical analysis
For all meta-analyses, the Bayesian Hierarchical model for Meta-Analysis was applied as presented in section 5.6 of Gelman to estimate the overall hazard ratio along with a central 95% credible interval. This model is the Bayesian version of the random-effects meta-analysis introduced by DerSimonian and Laird (1986) [5,6]. More specifically, for each study j, we performed the log of the hazard ratio, yj, and computed the standard deviation of the log hazard ratio estimate, sd(yj), based on the length of the log-transformed confidence intervals. We fit the Bayesian mode yj ~ Normal(θj = σj) with a standard deviation σj = sd(yj) and priors θj ~ Normal(µ, τ) and p(µ, τ) proportional to 1. For meta-analysis that considers four or fewer studies, priors p(µ) proportional to 1 and p(τ) a standard normal restricted to the positive real line was used. The overall hazard ratio estimate is the posterior expected value of exp (µ) with a central 95% credible interval given by the 0.025 and 0.975 posterior quantiles of exp (µ). To standardize notation, in this paper, we call p - value the Bayesian measure of evidence against the Null hypothesis (Pereira and Stern, 1999), defined as the smallest number ev such that the 1-ev central credible interval for estimate overall HR (i.e., exp(mu)) does not contain the null value (HR = 1) indicating overall no difference between the treatment groups [7]. The Bayesian measure of evidence is a p - value in the sense that it is the result of “inverting the credible interval” similar to the inversion of the confidence interval to compute a p - value. Additionally, as a measurement of heterogenicity in the outcomes across studies, we compute the Bayesian version of I2 that is interpreted as the total variation in the estimates of treatment effect that is due to heterogeneity between studies [8]. Values of I2 close to zero indicates that the clinical trials are homogeneous for the category while values close to 1 indicate heterogenicity [8].
p - values, for determination of statistical significance, were evaluated against an alpha value of 0.05. A p - value less than the alpha value of 0.05 was determined to be statistically significant and the null hypothesis of no difference between treatment and placebo was rejected. For the Ejection Fraction analysis, an analysis of variance was performed to detect the main effects, and differences between SGLT-2 inhibitors and GLP-1RA. Computations were carried out using R statistical programming language and R package rstan.
Major Adverse Cardiovascular Event (MACE)
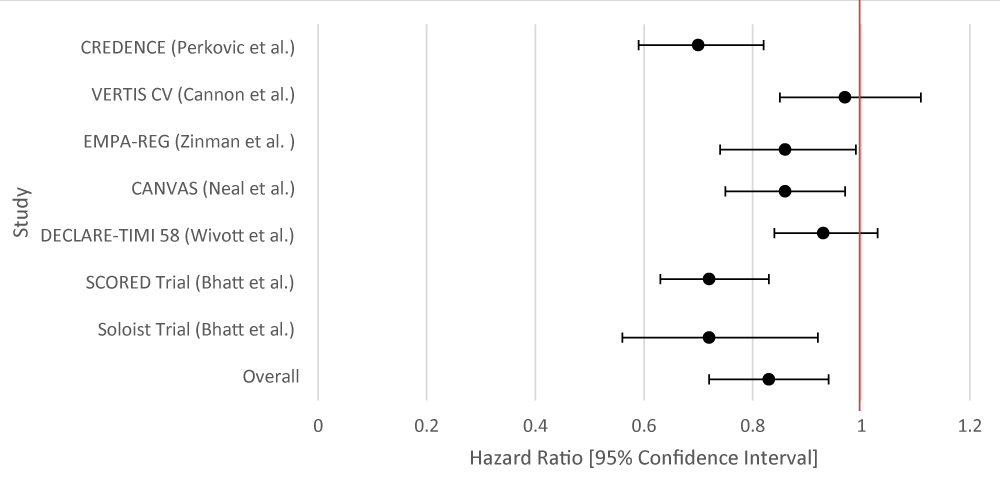
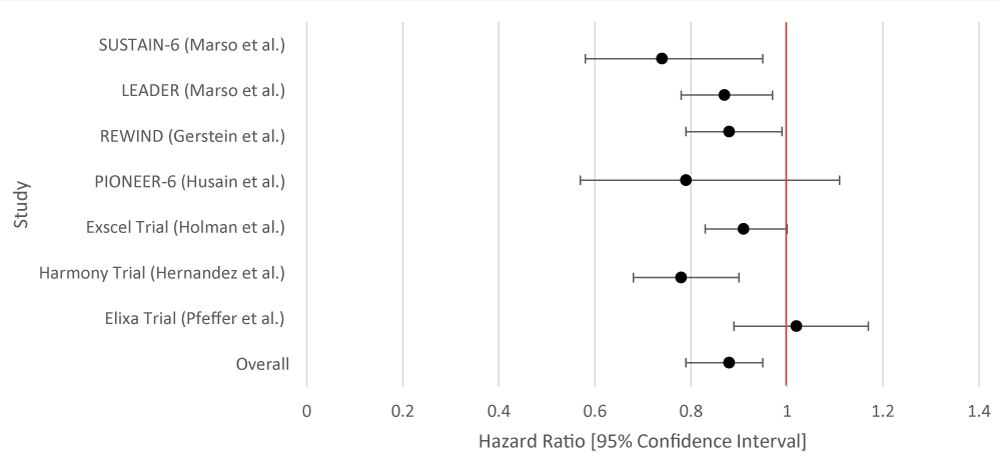
The overall aggregate of the studies demonstrated statistical significance for both SGLT-2i and GLP-1RA in the prevention of MACE. Both groups employed studies with large sample sizes. Even within individual studies, a majority of the studies that were included for each drug type demonstrated statistical significance with respect to MACE (Tables 1,2; Figures 1,2).
| Table 1: Data Results of Studies Investigating the Effect of SGLT-2i on Primary Cardiovascular Outcomes. | |||
| Author | Study Population | SGLT-2i Type | HR [95% Confidence Interval] |
| CREDENCE [9] | 4, 401 patients with T2DM and chronic kidney disease. | Canagliflozin | 0.70 [0.59 - 0.82] |
| VERTIS CV [10] | 8,246 randomly assigned patients with T2DM and atherosclerotic cardiovascular disease. | Ertugliflozin | 0.97 [0.85 - 1.11] |
| EMPA-REG [11] | 7,020 patients with T2DM at high cardiovascular risk. | Empagliflozin | 0.86 [0.74 - 0.99] |
| CANVAS 12 | 10,142 participants with T2DM and high cardiovascular risk. | Canagliflozin | 0.86 [0.75 - 0.97] |
| DECLARE-TIMI 58 [13] | 17,160 patients patients with T2DM who had or were at risk for atherosclerotic cardiovascular disease |
Dapagliflozin |
0.93 [0.84 - 1.03] |
| SCORED Trial [14] | 10, 584 patients were randomized in a 1:1 fashion to either sotagliflozin 400 mg daily (n = 5,292) or placebo (n = 5,292). Sotagliflozin was started at 200 mg daily and increased to the target dose if there were no unacceptable side effects. | Sotagliflozin | 0.72 [0.63 - 0.83] |
| Soloist Trial [15] | 1,222 patients with T2DM who were recently hospitalized for worsening heart failure were randomly assigned to receive sotagliflozin or placebo. | Sotaglifozin | 0.72 [0.56 - 0.92] |
| Overall Results | 0.83 [0.72 - 0.94] | ||
| p – value | 0.014 | ||
| I2 | 76.68 | ||
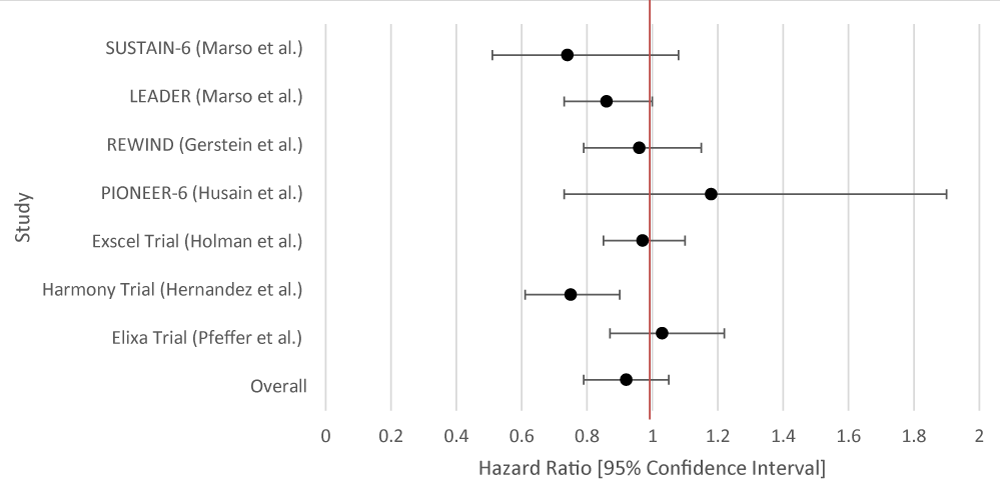
| Table 2: Data Results of Studies Investigating the Effect of GLP-1RA on Primary Cardiovascular Outcomes. | |||
| Author or Trial | Study Population | GLP-1RA | Hazard Ratio [95% Confidence Interval] |
| SUSTAIN-6 [16] | 3,297 patients with T2DM and glycated Hgb 7% or more. | Semaglutide | 0.74 [0.58 - 0.95] |
| LEADER 17 | 9,340 patients were randomly assigned patients with T2DM and high cardiovascular risk to receive liraglutide or placebo. | Liraglutide | 0.87(0.78 - 0.97) |
| REWIND [18] | 9,901 patients at least 50 years old with T2DM who had either a previous cardiovascular event or cardiovascular risk factors. | Dulaglutide | 0.88 [0.79 - 0.99] |
| PIONEER-6 [19] | Total: 5,878 patients. 3,183 patients. The mean age of the patients was 66 years; 2695 patients (84.7%) were 50 years of age or older and had cardiovascular or chronic kidney disease. | Semaglutide | 0.79 [0.57 - 1.11] |
| Exscel Trial [20] | 14,752 patients with T2DM regardless of previous cardiovascular disease. | Exenatide | 0.91 [0.83 - 1.00] |
| Harmony Trial [21] | Total: 9,463 patients. 4,731 patients were assigned to receive albiglutide and 4,732 patients to receive placebo. Patient ages were 40 years and older. Underlying conditions included T2DM and cardiovascular disease (at a 1:1 ratio). | Albiglutide | 0.78 [0.68 - 0.90] |
| Elixa Trial [22] | 6,068 patients with T2DM who had had a myocardial infarction or who had been hospitalized for unstable angina within the previous 180 days to receive lixisenatide or placebo | Lixisenatide | 1.02 [0.89 - 1.17] |
| Overall Results | 0.88 [0.79 - 0.95] | ||
| p - value | 0.012 | ||
| I2 | 72.44 | ||
Figure 1: Forest Plot of Studies Investigating the Effect of SGLT-2i on Primary Cardiovascular Outcomes.
Figure 2: Forest Plot of Studies Investigating the Effect of GLP-1RA on Primary Cardiovascular Outcomes.
Heart failure hospitalizations
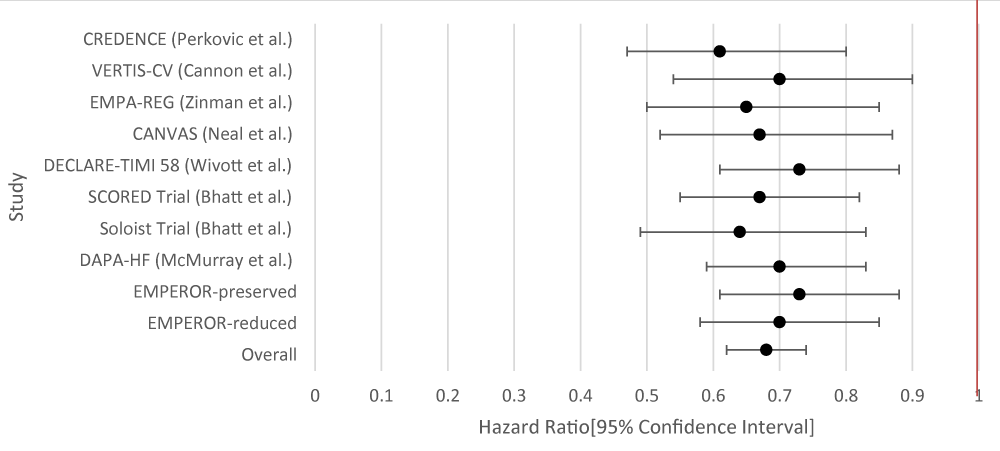
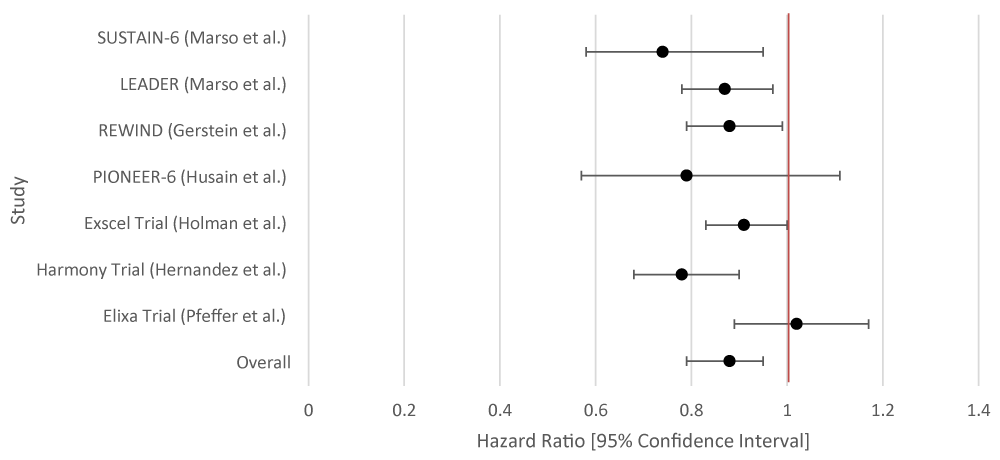
The overall aggregate of the studies demonstrated statistical significance for SGLT-2i. GLP-1RA did not demonstrate statistical significance. Within individual studies, all of the studies for SGLT-2i showed a statistically significant result. For GLP-1RA only one such study demonstrated a benefit (Tables 3,4; Figures 3,4).
| Table 3: Data Results of Studies Investigating the Effect of SGLT-2i on HF Hospitalizations. | |||
| Author | Study Population | SGLT-2i Type | Hazard Ratio [95% Confidence Interval] |
| CREDENCE [9] | 4,401 patients with T2DM and Chronic Kidney Disease (CKD). | Canagliflozin | 0.61 [0.47 - 0.80] |
| VERTIS-CV [10] | 8,246 patients with T2DM and atherosclerotic cardiovascular disease. | Ertugliflozin | 0.70 [0.54 - 0.90] |
| EMPA-REG [11] | 7,020 patients with T2DM at high cardiovascular risk. | Empagliflozin | 0.65 [0.5 - 0.85] |
| CANVAS [12] | 10,142 participants with T2DM and high cardiovascular risk. Participants in each trial were randomly assigned to receive canagliflozin or placebo. | Canagliflozin | 0.67 [0.52 - 0.87] |
| DECLARE-TIMI 58 [13] | 17,160 patients with T2DM had multiple risk factors for atherosclerotic cardiovascular disease or had established atherosclerotic cardiovascular disease. | Dapagliflozin | 0.73 [0.61 - 0.88] |
| SCORED Trial [14] | 10, 584 patients were randomized in a 1:1 fashion to either sotagliflozin 400 mg daily (n = 5,292) or placebo (n = 5,292). Sotagliflozin was started at 200 mg daily and increased to the target dose if there were no unacceptable side effects. | Sotagliflozin | 0.67 [0.55 - 0.82] |
| Soloist Trial [15] | 1,222 patients with T2DM who were recently hospitalized for worsening heart failure were randomly assigned to receive sotagliflozin or placebo. | Sotaglifozin | 0.64 [0.49 - 0.83] |
| DAPA-HF [23] | 4,744 patients with New York Heart Association class II, III, or IV heart failure and an ejection fraction of 40% or less to receive either dapagliflozin (at a dose of 10 mg once daily) or placebo. | Dapagliflozin | 0.70 [0.59 - 0.83] |
| Emperor-Preserved [24] | 5,988 patients with class II-IV heart failure and an ejection fraction of more than 40% to receive empagliflozin or placebo. | Empagliflozin | 0.73 [0.61 - 0.88] |
| Emperor-Reduced [25] | 3,730 patients with class II, III, or IV heart failure and an ejection fraction of 40% or less to receive empagliflozin (10 mg once daily) or placebo | Empaglifozin | 0.70 [0.58 - 0.85] |
| Overall Results | 0.69 [0.64 - 0.74] | ||
| p - value | < 0.001 | ||
| I2 | 25.81 | ||
| Table 4: Data Results of Studies Investigating the Effect of GLP-1RA on HF Hospitalizations. | |||
| Author or Trial | Study Population | GLP-1RA | Hazard Ratio [95% Confidence Interval] |
| SUSTAIN-6 [16] | 3,297 patients with T2DM and glycated Hgb 7% or more. | Semaglutide | 1.11 [0.77 - 1.61] |
| LEADER [17] | 9,340 patients randomized who had T2DM. | Liraglutide | 0.87 [0.73 - 1.05] |
| REWIND [18] | 9,901 patients at least 50 years old with T2DM who had either a previous cardiovascular event or cardiovascular risk factors. | Dulaglutide | 0.93 [0.77 - 1.12] |
| PIONEER-6 [19] | Total: 5,878 patients. 3,183 patients. The mean age of the patients was 66 years; 2695 patients (84.7%) were 50 years of age or older and had cardiovascular or chronic kidney disease. | Semaglutide | 0.86 [ 0.48 - 1.55] |
| Exscel Trial [20] | 14,752 patients with T2DM regardless of previous cardiovascular disease. | Exenatide | 0.94 [0.70 - 1.13] |
| Harmony Trial [21] | Total: 9,463 patients. 4,731 patients were assigned to receive albiglutide and 4,732 patients to receive placebo. Patient ages were 40 years and older. Underlying conditions included T2DM and cardiovascular disease (at a 1:1 ratio). |
Albiglutide | 0.71 [0.53 - 0.94] |
| Elixa Trial [22] | 6,068 patients with T2DM who had had an MI or who had been hospitalized for unstable angina within the previous 180 days received lixisenatide or placebo. | Lixisenatide | 0.96 [0.75 - 1.23] |
| Overall Results | 0.90 [0.79 - 1.04] | ||
| p - value | 0.122 | ||
| I2 | 48.64 | ||
Figure 3: Forest Plot of Results of Studies Investigating the Effect of SGLT-2i on HF Hospitalizations.
Figure 4: Forest Plot of Studies Investigating the Effect of GLP-1RA on HF Hospitalizations.
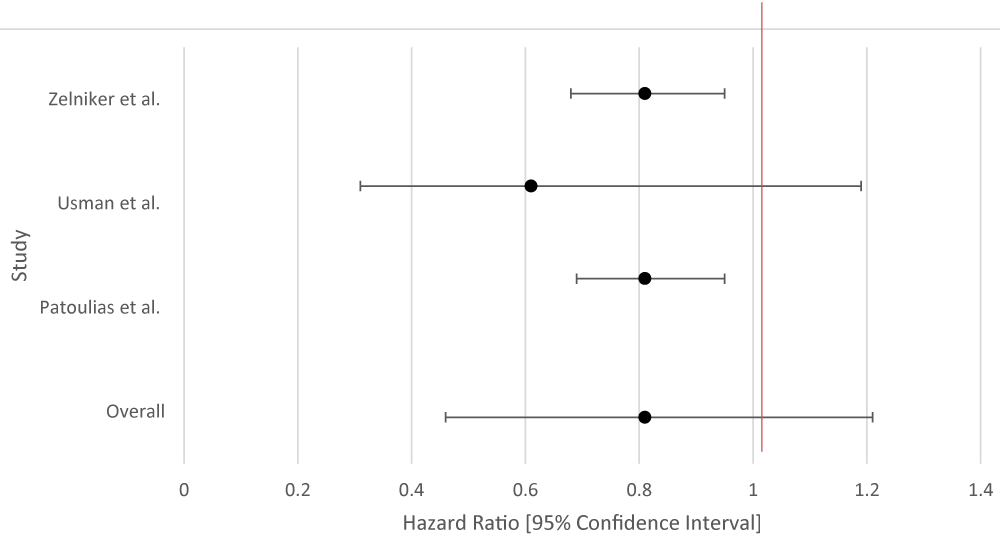
The overall aggregate of the studies demonstrated statistical significance for both SGLT-2i and GLP-1RA in the prevention of MACE. Both groups employed studies with large sample sizes. Even within individual studies, a majority of the studies that were included for each drug type demonstrated Z2/3 of the studies did show benefit. Data for GLP-1RA were limited (Table 5; Figure 5).
| Table 5: Data Results of Studies Investigating the Effect of SGLT-2i on Atrial Fibrillation. | |||
| Author | Study Population | SGLT-2i Type | Hazard Ratio [95% Confidence Interval] |
| Zelniker, et al. [26] | 17, 160 patients with T2DM and multiple risk factors for atherosclerotic cardiovascular disease. | Dapagliflozin | 0.81 [0.68 - 0.95] |
| Usman, et al. [27] | 35 randomized controlled trials with a total of 34,987 patients with T2DM. | Unspecified SGLT-2 | 0.61 [0.31 - 1.19] |
| Patoulias, et al. [28] | Patients with T2DM and Cardiac Risk Factors | SGLT-2 inhibitors (unspecified) |
0.81 [0.69 - 0.95] |
| Overall Results | 0.81 [0.46 - 1.24] | ||
| p -value | 0.144 | ||
| I2 | 92.47 | ||
Figure 5: Forest Plot of Studies Investigating the Effect of SGLT-2i on Atrial Fibrillation.
Stroke
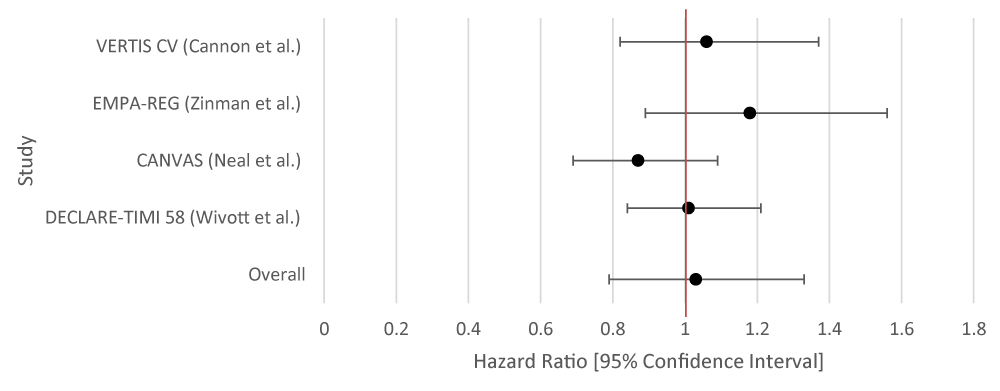
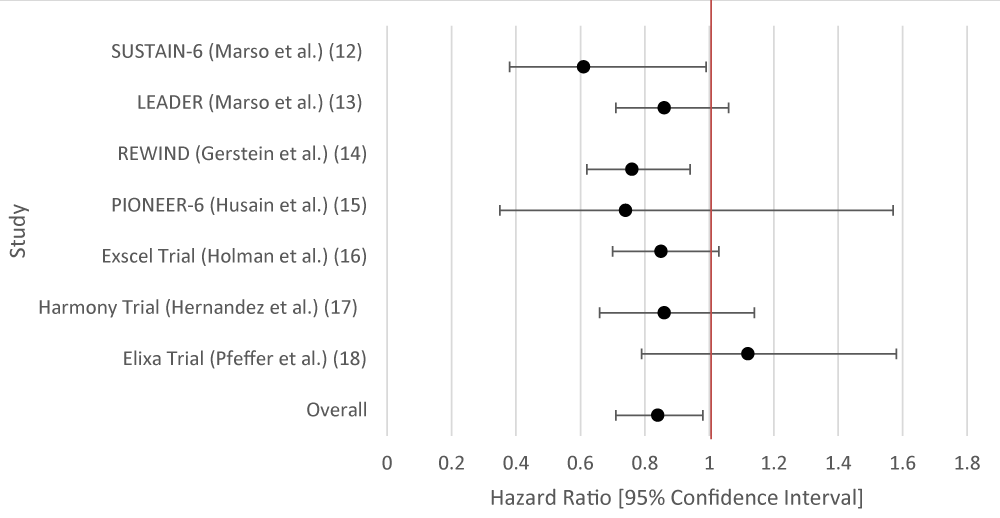
The overall aggregate of studies for GLP-1RA demonstrated statistically significant benefits in preventing stroke, but not SGLT-2 inhibitors. Individually, three of the studies for GLP-1RA showed benefit with respect to stroke reduction while none of the studies for SGLT-2i demonstrated statistical significance (Tables 6,7; Figures 6,7).
| Table 6: Data Results of Studies Investigating the Effect of SGLT-2i on Stroke. | |||
| Author | Study Population | SGLT-2i Type | Hazard Ratio [95% Confidence Interval] |
| VERTIS CV [10] | 8,246 randomly assigned patients with T2DM and atherosclerotic cardiovascular disease. | Ertugliflozin | 1.06 [0.82 - 1.37] |
| EMPA-REG [11] | 7, 028 patients with T2DM. | Empagliflozin | Fatal or Nonfatal Stroke: 1.18 [0.89 - 1.56] |
| CANVAS [12] | 9,734 participants with T2DM. |
Canagliflozin | 0.87 [0.69 - 1.09] |
| DECLARE-TIMI 58 [13] | 17,160 patients with T2DM and had multiple risk factors for atherosclerotic cardiovascular disease or had established atherosclerotic cardiovascular disease | Dapagliflozin | 1.01 [0.84 - 1.21] |
| Overall Results | 1.03 [0.79 - 1.33] | ||
| p - value | 0.869 | ||
| I2 | 81.43 | ||
| Table 7: Data Results of Studies Investigating the Effect of GLP-1RA on Stroke. | |||
| Author or Trial | Study Population | GLP-1RA | Hazard Ratio [95% Confidence Interval] |
| SUSTAIN-6 [16] | Randomized, double-blind, placebo-controlled, parallel-group trial; 3,297 patients with T2DM and glycated Hgb 7% or more. Patients were randomized in a 1:1:1:1 ratio to receive either 0.5 mg or 1.0 mg of once-weekly subcutaneous semaglutide or volume-matched placebo. | Semaglutide | 0.61 [0.38 - 0.99] |
| LEADER [17] | 9,340 patients randomized who had T2DM (glycated hemoglobin of 7% or higher, n = 4,668 liraglutide group, n = 4,672 placebo group | Liraglutide | 0.86 [0.71 - 1.06] |
| REWIND [ 18] | 9,901 patients at least 50 years old with T2DM who had either a previous cardiovascular event or cardiovascular risk factors. | Dulaglutide | 0.76 [0.62 - 0.94] |
| PIONEER-6 [19] | 3, 183 patients. The mean age of the patients was 66 years; 2695 patients (84.7%) were 50 years of age or older and had cardiovascular or chronic kidney | Semaglutide | 0.74 [0.35 - 1.57] |
| Exscel Trial [20] | 14,752 patients with T2DM regardless of previous cardiovascular disease. | Exenatide | Total Stroke: 0.85 [0.70 - 1.03] |
| Harmony Trial [21] | 4,731 patients were assigned to receive albiglutide and 4,732 patients to receive placebo. Patient ages were 40 years and older. Underlying conditions included T2DM and cardiovascular disease (at a 1:1 ratio). | Albiglutide | 0.86 [0.66 - 1.14] |
| Elixa Trial [22] | 6,068 patients with T2DM who had had a myocardial infarction or who had been hospitalized for unstable angina within the previous 180 days received lixisenatide or placebo. | Lixisenatide | 1.12 [0.79 - 1.58] |
| Overall Results | 0.84 [0.71 - 0.97] | ||
| p - value | 0.029 | ||
| I2 | 53.91 | ||
Figure 6: Forest Plot of Studies Investigating the Effect of SGLT-2i on Stroke.
Figure 7: Forest Plot of Studies Investigating the Effect of GLP-1RA on Stroke.
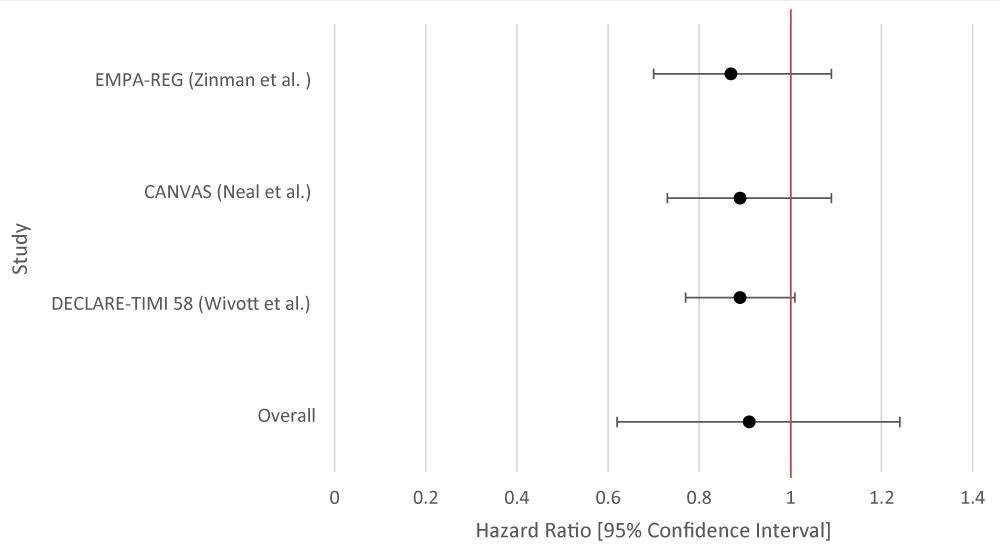
Myocardial infarction
No statistically significant benefit for either SGLT-2i or GLP-1RA. Individually, none of the studies demonstrated statistical significance for SGLT-2i while only one of the studies for GLP-1RA demonstrated statistical significance (Tables 8,9; Figures 8,9).
Figure 8: Forest Plot of Studies Investigating the Effect of SGLT-2i on Myocardial Infarction.
Figure 9: Forest Plot of Studies Investigating the Effect of GLP-1RA on Myocardial Infarction.
| Table 8: Data Results of Studies Investigating the Effect of SGLT-2i on Myocardial Infarction. | |||
| Author | Study Population | SGLT-2i Type | Hazard Ratio [95% Confidence Interval] |
| EMPA-REG [ 11] | 7, 028 patients with T2DM | Empagliflozin | 0.87 [0.70 - 1.09] |
| CANVAS [12] | 9,734 participants with T2DM |
Canagliflozin | 0.89 [0.73 - 1.09] |
| DECLARE-TIMI 58 [13] | 17,160 patients with T2DM had multiple risk factors for atherosclerotic cardiovascular disease or had established atherosclerotic cardiovascular disease. | Dapagliflozin | 0.89 [0.77 - 1.01] |
| Overall Results | 0.90 [0.65 - 1.21] | ||
| p - value | 0.213 | ||
| I2 | 90 | ||
| Table 9: Data Results of Studies Investigating the Effect of GLP-1RA on Myocardial Infarction. | |||
| Author or Trial | Study Population | GLP-1RA | Hazard Ratio [95% Confidence Interval] |
| Marso, et al. [16] | 3,297 patients with T2DM and glycated Hgb 7% or more. | Semaglutide | Nonfatal: 0.74 [0.51 - 1.08] |
| Marso, et al. [17] | 9,340 patients randomized who had T2DM | Liraglutide | 0.86 [0.73 - 1.00] |
| REWIND [18] | Randomized, double-blind, placebo-controlled trial. 9,901 Men and women aged at least 50 years with T2DM who had either a previous cardiovascular event or cardiovascular risk factors were randomly assigned (1:1) to either weekly subcutaneous injection of dulaglutide (1.5 mg) or placebo. | Dulaglutide | 0.96 [0.79 - 1.15] |
| PIONEER-6 [19] | 3183 patients were randomly assigned to receive oral semaglutide or placebo. The mean age of the patients was 66 years; 2695 patients (84.7%) were 50 years of age or older and had cardiovascular or chronic kidney disease. | Semaglutide | 1.18 [0.73 - 1.90] |
| Exscel Trial [20] | 14,752 patients with T2DM were randomly assigned, with or without previous cardiovascular disease, to receive subcutaneous injections of extended-release exenatide at a dose of 2 mg or matching placebo once weekly. Patients were followed for a median of 3.2 years (interquartile range, 2.2 to 4.4). | Exenatide | 0.97 [0.85 - 1.10] |
| Harmony Trial [21] | Total: 9,463 patients. Double-blind, randomized, placebo-controlled trial: 4731 patients were assigned to receive albiglutide and 4732 patients to receive placebo; patients aged 40 years and older with T2DM and cardiovascular disease (at a 1:1 ratio) to groups that either received a subcutaneous injection of albiglutide (30–50 mg, based on glycemic response and tolerability) or of a matched volume of placebo once a week, in addition to their standard care. |
Albiglutide | 0.75 [0.61 - 0.90 |
| Elixa Trial [22] | 6,068 patients with T2DM who had had a myocardial infarction or who had been hospitalized for unstable angina within the previous 180 days to receive lixisenatide or placebo | Lixisenatide | 1.03 [0.87 - 1.22] |
| Overall Results | 0.92 [0.79 - 1.05] | ||
| p - value | 0.147 | ||
| I2 | 70.08 | ||
Heart failure symptoms (Table 10)
| Table 10: Data Results of Studies Investigating the Effect of SGLT-2i on HF Symptoms. | |||
| Author | Study Population | SGLT-2i Type | Change in HF symptoms |
| Ingelheim, et al. [29] | 315 participants with T2DM | Empagliflozin | Exercise Capacity score was 299.5 for the placebo vs 297.0 for the Empagliflozin group |
| Abraham, et al. [30] | HF patients with and without T2DM | Empagliflozin | +2.7% in 6MWTD (Dyspnea Score) |
| Nunez, et al. [31] | Nineteen patients with T2DM and symptomatic HF | Empagliflozin | +11.1% in peak VO21 |
This meta-analysis of the current landmark trial data regarding the utility of SGLT-2i and GLP-1RA explored the efficacy of both anti-hyperglycemic medications in the reduction of cardiovascular conditions and symptoms while also exploring differences between the two medication classes. Our findings are consistent with other published studies and confirm the various cardioprotective effects of these medications. Moreover, in this analysis, we studied varying efficacies of different medications in either of the drug classes in preventing MACE (major adverse cardiovascular events), heart failure hospitalizations, atrial fibrillation, stroke, and myocardial infarction. Additionally, this analysis studied the ability of both medications in either drug class to improve heart failure symptoms.
This study had two major findings: both medication classes help prevent Major Cardiovascular Events (MACE) and the patient’s underlying conditions should not necessarily guide which class of medication to choose.
Regarding MACE, analysis of the above trials of SGLT-2i and GLP-1RA clearly demonstrated that both anti-hyperglycemic had a statistically significant reduction in the incidence of major adverse cardiovascular events. A similar magnitude of reduction in MACE has been noticed in previously published studies comparing the two drug class [32]. This shows that these medications can be of major benefit to patients at risk of major cardiovascular events. Cardiac events are among the leading causes of death. As a result, these results mean that these classes of medications can be among the leading agents in the prevention of death overall.
Regarding individual effects, SGLT-2i and GLP-1RA do not appear to have much in the way of preventing individual conditions, with the exception of SGLT-2i with respect to Heart Failure and GLP-1RA with respect to stroke. Furthermore, this means that with regard to atrial fibrillation, stroke, and myocardial infarction, there is no demonstrated improvement in either class of medication.
This suggests that individual conditions should not be the primary focus of these medications. Preventing overall cardiovascular outcomes should be the primary focus of prescribing either SLGT-2 inhibitors or GLP-1RA. If a patient has either heart failure or pre-disposition to stroke, it may be then wise to prescribe SGLT-2i or GLP-1RA agonists, respectively. It also means that more studies on alternative agents for preventing atrial fibrillation, stroke, or myocardial infarction must be pursued.
On the topic of heart failure hospitalizations, there was a striking difference noticed in terms of heart failure hospitalization rates with the use of SGLT-2i compared with GLP-1RA agonists. This is consistent with results from a large population-based analysis by Patorno, et al. in which the initiation of SGLT-2i versus GLP-1RA therapy was associated with an approximately 30% reduction in the risk for the primary HHF outcome in all included patients, regardless of the presence or absence of cardiovascular disease at baseline [33]. This finding is also consistent with the effects of SGLT2 inhibitors on heart failure hospitalizations in cardiovascular outcome trials and in other real-world comparisons to other antidiabetic drugs [34].
Multiple mechanisms have been proposed for the cardioprotective effects of SGLT-2i, while the mechanism for the cardioprotective effects of GLP-1RA is still relatively unknown. Lopaschuk and Verma proposed that SGLT-2i increases diuresis, decreases blood pressure, and decreases sympathetic response [35]. This may translate into reduced cardiac afterload and improved ventricular-atrial coupling, possibly explaining why SGLT-2i performs better in reducing heart failure hospitalizations and improving heart failure symptoms [34]. Cox, et al. demonstrated that GLP-1RA reduces blood sugar levels and indirectly reduces inflammation or atherosclerotic plaque formation [36]. Given that atherosclerotic plaque formation and rupture are among the foundational causes of strokes, this can be hypothesized to explain a reduction in stroke events seen in patients on GLP-1RA agnostic therapy.
This meta-analysis includes a diverse number of studies and presents a holistic picture of how SGLT-2i and GLP-1RA perform in the general cardiovascular risk population. This study may be particularly useful for clinicians needing to decide on appropriate treatment regimens for patients with diabetes and concurrent cardiovascular symptoms. However, this study still has certain limitations. First, the availability of data on GLP-1RA was limited compared to the data available on SGLT-2 inhibitors, making some comparison computations heterogeneous. Second, the current study suffers from the inherent limitation of meta-analysis that can arise as the positive results might be published more easily than the negative results. Thirdly, there could be other confounding variables present with regard to test subjects. Dietary habits, lifestyle differences, and inherent genetic differences all are factors that are difficult to control for in a randomized controlled trial. Consequently, it is difficult to say that the treatment alone accounts for differences present between treatment and placebo groups. Additionally, the number of trials included in SGLT2 inhibitors treatment and GLP‐1RA treatment might not be comparable. Therefore, more complete data must be available in order to create a more thorough meta-analysis.
The data of this meta-analysis suggests that both SGLT-2i and GLP-1RA are important considerations in the medication repertoire for physicians treating patients with diabetes, heart failure, those at risk for a major cardiovascular event, or a combination of these conditions. These meta-analyses demonstrate that both SGLT-2i and GLP-1RA provide statistically significant benefits in a diverse array of cardiovascular conditions. Both classes of anti-hyperglycemic provide protection against major cardiovascular events. With regard to specific conditions, SGLT-2i demonstrates greater benefit in patients with heart failure, while GLP-1RA demonstrates greater benefit in patients at risk for a stroke.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
1. AJ obtained, analyzed, and interpreted the data. AJ participated in the statistical analysis. AJ was a major contributor to writing the manuscript.
2. KH obtained, analyzed, and interpreted the data. KH participated in the statistical analysis. KH was a major contributor to writing the manuscript.
3. TA obtained, analyzed, and interpreted the data. TA participated in the statistical analysis. TA was a major contributor to writing the manuscript.
4. LLN obtained, analyzed, and interpreted the data. LLN participated in the statistical analysis. LLN was a major contributor to writing the manuscript.
5. TK obtained, analyzed, and interpreted the data. TK participated in the statistical analysis. TK was a major contributor to writing the manuscript.
6. All authors read and approved the final manuscript.
- World Health Organization. (2021, June 11). Cardiovascular Diseases (CVDs) Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023 Feb 21;147(8):e93-e621. doi: 10.1161/CIR.0000000000001123. Epub 2023 Jan 25. Erratum in: Circulation. 2023 Feb 21;147(8):e622. Erratum in: Circulation. 2023 Jul 25;148(4):e4. PMID: 36695182.
- Carter HE, Schofield D, Shrestha R. Productivity costs of cardiovascular disease mortality across disease types and socioeconomic groups. Open Heart. 2019 Feb 16;6(1):e000939. doi: 10.1136/openhrt-2018-000939. PMID: 30997129; PMCID: PMC6443138.
- CDC – National Center for Chronic Disease Prevention and Health Promotion, Division for Heart Disease and Stroke Prevention – Heart Disease Facts. (2023) https://www.cdc.gov/heartdisease/facts.htm (Accessed: 23 August 2023).
- Lafeber M, Grobbee DE, Spiering W, van der Graaf Y, Bots ML, Visseren FL; SMART Study Group. The combined use of aspirin, a statin, and blood pressure-lowering agents (polypill components) in clinical practice in patients with vascular diseases or type 2 diabetes mellitus. Eur J Prev Cardiol. 2013 Oct;20(5):771-8. doi: 10.1177/2047487312449587. Epub 2012 May 30. PMID: 22649123.
- Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr, Sperling LS, Virani SS, Blumenthal RS. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019 Jun 25;73(24):3153-3167. doi: 10.1016/j.jacc.2018.11.005. Epub 2018 Nov 10. Erratum in: J Am Coll Cardiol. 2019 Jun 25;73(24):3234. PMID: 30423392.
- Grundy SM, Stone NJ; Guideline Writing Committee for the 2018 Cholesterol Guidelines. 2018 Cholesterol Clinical Practice Guidelines: Synopsis of the 2018 American Heart Association/American College of Cardiology/Multisociety Cholesterol Guideline. Ann Intern Med. 2019 Jun 4;170(11):779-783. doi: 10.7326/M19-0365. Epub 2019 May 28. PMID: 31132793.
- Whelton PK, Carey RM. The 2017 Clinical Practice Guideline for High Blood Pressure. JAMA. 2017 Dec 5;318(21):2073-2074. doi: 10.1001/jama.2017.18209. PMID: 29159375.
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Sep 10;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678. Epub 2019 Mar 17. Erratum in: Circulation. 2019 Sep 10;140(11):e649-e650. Erratum in: Circulation. 2020 Jan 28;141(4):e60. Erratum in: Circulation. 2020 Apr 21;141(16):e774. PMID: 30879355; PMCID: PMC7734661.
- Arif H, Aggarwal S. Salicylic Acid (Aspirin) [Updated 2023 Jul 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK519032/
- Capodanno D, Angiolillo DJ. Aspirin for Primary Cardiovascular Risk Prevention and Beyond in Diabetes Mellitus. Circulation. 2016 Nov 15;134(20):1579-1594. doi: 10.1161/CIRCULATIONAHA.116.023164. Epub 2016 Oct 11. PMID: 27729421.
- Weisman SM, Brunton S. Primary Prevention of CVD with Aspirin: Benefits vs Risks. J Fam Pract. 2021 Jul;70(6S):S41-S46. doi: 10.12788/jfp.0222. PMID: 34432623.
- Tai FWD, McAlindon ME. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin Med (Lond). 2021 Mar;21(2):131-134. doi: 10.7861/clinmed.2021-0039. PMID: 33762373; PMCID: PMC8002800.
- Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017 Aug 5;390(10094):613-624. doi: 10.1016/S0140-6736(16)32404-7. Epub 2017 Feb 25. PMID: 28242110.
- US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, Cabana M, Chelmow D, Coker TR, Davis EM, Donahue KE, Jaén CR, Krist AH, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Tseng CW, Wong JB. Aspirin Use to Prevent Cardiovascular Disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022 Apr 26;327(16):1577-1584. doi: 10.1001/jama.2022.4983. PMID: 35471505.
- McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, Shah RC, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Johnston CI, Ryan J, Radziszewska B, Jelinek M, Malik M, Eaton CB, Brauer D, Cloud G, Wood EM, Mahady SE, Satterfield S, Grimm R, Murray AM; ASPREE Investigator Group. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018 Oct 18;379(16):1509-1518. doi: 10.1056/NEJMoa1805819. Epub 2018 Sep 16. PMID: 30221597; PMCID: PMC6289056.
- Zheng SL, Roddick AJ. Association of Aspirin Use for Primary Prevention With Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-analysis. JAMA. 2019 Jan 22;321(3):277-287. doi: 10.1001/jama.2018.20578. Erratum in: JAMA. 2019 Jun 11;321(22):2245. PMID: 30667501; PMCID: PMC6439678.
- Guirguis-Blake JM, Evans CV, Senger CA, O'Connor EA, Whitlock EP. Aspirin for the Primary Prevention of Cardiovascular Events: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016 Jun 21;164(12):804-13. doi: 10.7326/M15-2113. Epub 2016 Apr 12. PMID: 27064410.
- Wang M, Yu H, Li Z, Gong D, Liu X. Benefits and Risks Associated with Low-Dose Aspirin Use for the Primary Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Control Trials and Trial Sequential Analysis. Am J Cardiovasc Drugs. 2022 Nov;22(6):657-675. doi: 10.1007/s40256-022-00537-6. Epub 2022 May 16. PMID: 35570250.
- Dehmer SP, O’Keefe LR, Grossman ES. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: An Updated Decision Analysis for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2022 Apr. (Technical Report, No. 211s.) https://www.ncbi.nlm.nih.gov/books/NBK580862/
- Dehmer SP, O'Keefe LR, Evans CV, Guirguis-Blake JM, Perdue LA, Maciosek MV. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2022 Apr 26;327(16):1598-1607. doi: 10.1001/jama.2022.3385. PMID: 35471506.
- Akintoye E, Afonso L, Bengaluru Jayanna M, Bao W, Briasoulis A, Robinson J. Prognostic Utility of Risk Enhancers and Coronary Artery Calcium Score Recommended in the 2018 ACC/AHA Multisociety Cholesterol Treatment Guidelines Over the Pooled Cohort Equation: Insights From 3 Large Prospective Cohorts. J Am Heart Assoc. 2021 Jun 15;10(12):e019589. doi: 10.1161/JAHA.120.019589. Epub 2021 Jun 7. PMID: 34092110; PMCID: PMC8477885.