More Information
Submitted: March 22, 2024 | Approved: April 01, 2024 | Published: April 02, 2024
How to cite this article: Bianchi R, Esposito GM, Ciccarelli G, Tartaglione D, Golino P. A Complex Case with a Completely Percutaneous Solution: Treatment of a Severe Calcific Left Main in a Patient with Low-Flow Low-Gradient Aortic Stenosis. J Cardiol Cardiovasc Med. 2024; 9: 061-066.
DOI: 10.29328/journal.jccm.1001180
Copyright License: © 2024 Bianchi R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Coronary artery disease; Aortic stenosis; TAVI; Left atrial appendage Closure; Complex High-risk Coronary Procedure (CHIP); Ventricular support; Rotational atherectomy
A Complex Case with a Completely Percutaneous Solution: Treatment of a Severe Calcific Left Main in a Patient with Low-Flow Low-Gradient Aortic Stenosis
Renatomaria Bianchi*, Giovanni Marco Esposito, Giovanni Ciccarelli, Donato Tartaglione and Paolo Golino
Department of Cardiology, University of Campania, “Luigi Vanvitelli” Monaldi Hospital, Naples, Italy
*Address for Correspondence: Renatomaria Bianchi, MD, Department of Cardiology, University of Campania, “Luigi Vanvitelli” Monaldi Hospital, Naples, Italy, Email: [email protected]
Background: This case study explores an integrated approach to managing a complex cardiac condition, presenting a comprehensive single-session intervention. This includes balloon valvuloplasty using a Nucleus 18 mm balloon, complex angioplasty with rotational atherectomy (rotablator) targeting calcified lesions in the left main and left anterior descending artery, and Transcatheter Aortic Valve Implantation (TAVI) with a 23 mm Sapien 3 valve, all performed on an 81-year-old woman. Furthermore, this report underscores the strategic left atrial appendage closure conducted three months post-procedure due to the patient’s elevated hemorrhagic risk.
Case presentation: Facing critical coronary and valvular pathologies, the patient underwent a meticulously planned, single-session intervention. The process began with a balloon valvuloplasty using a Nucleus 18 mm balloon to address the aortic stenosis. This was followed by a high-risk angioplasty, during which the Impella CP device provided hemodynamic support and rotational atherectomy was employed to address the calcified coronary artery disease effectively. The same session saw the successful execution of TAVI using a 23 mm Sapien 3 valve. The comprehensive approach notably diminished procedural complications, illustrating the benefits of an integrated treatment pathway in managing high-risk patients. Three months later, the patient underwent a left atrial appendage closure, a critical move considering her high risk of hemorrhage. This procedure also provided an opportunity to assess the favorable outcomes of the previous angioplasty.
Conclusion: This case validates the feasibility and efficacy of performing multiple advanced percutaneous interventions in a single session for high-risk cardiac patients. It underscores the crucial role of innovative and personalized treatment strategies in improving patient outcomes, particularly in complex clinical scenarios. Moreover, the case exemplifies the essential relationship between immediate, comprehensive intervention and subsequent follow-up procedures in ensuring optimal long-term patient care.
The management of complex cardiac conditions in elderly patients often requires innovative and multifaceted treatment approaches. The integration of balloon valvuloplasty, protected high-risk angioplasty, and TAVI in a single session represents a significant advancement in interventional cardiology, especially for patients with multiple comorbidities and high procedural risk. Subsequent procedures like left atrial appendage closure further tailor the treatment to individual patient needs, especially in the context of bleeding risk management.
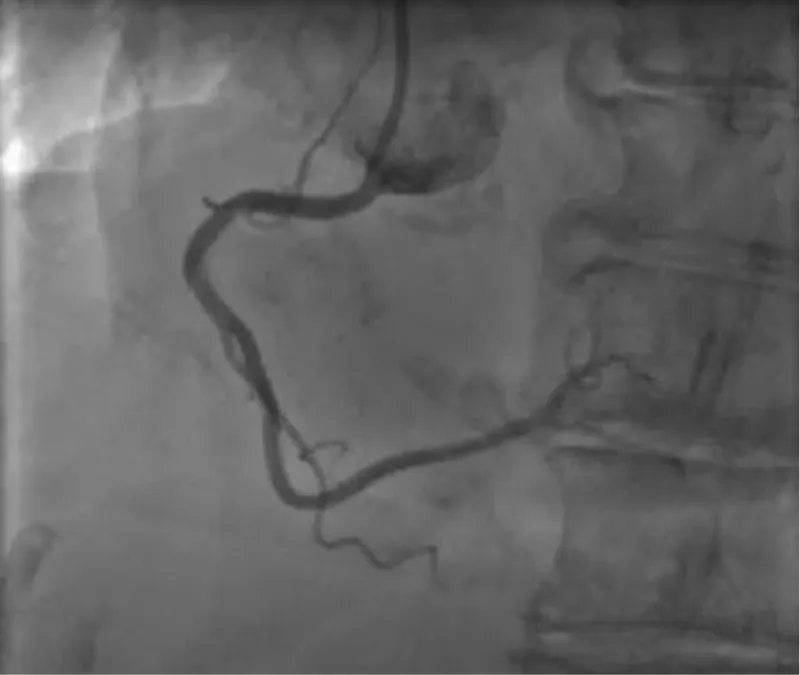
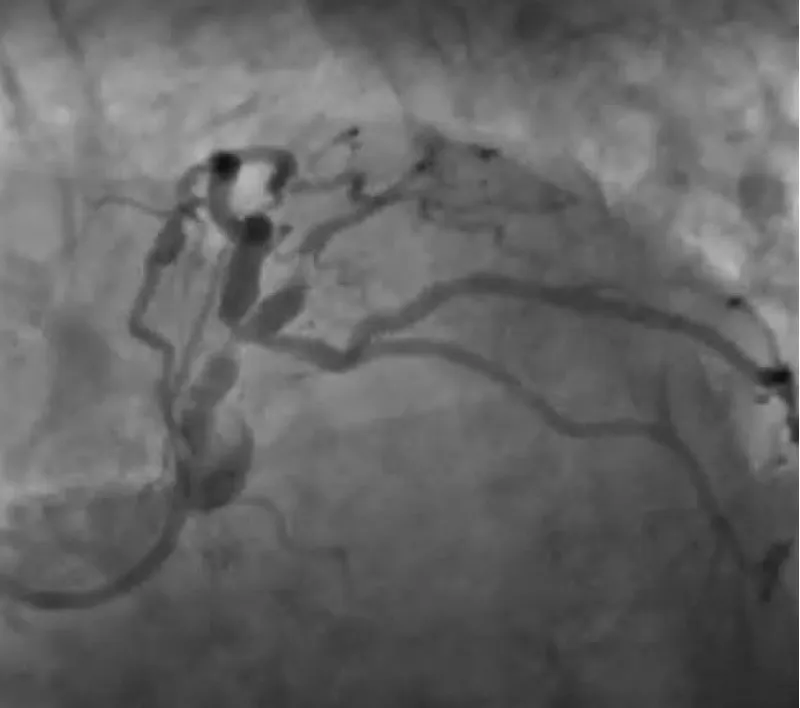
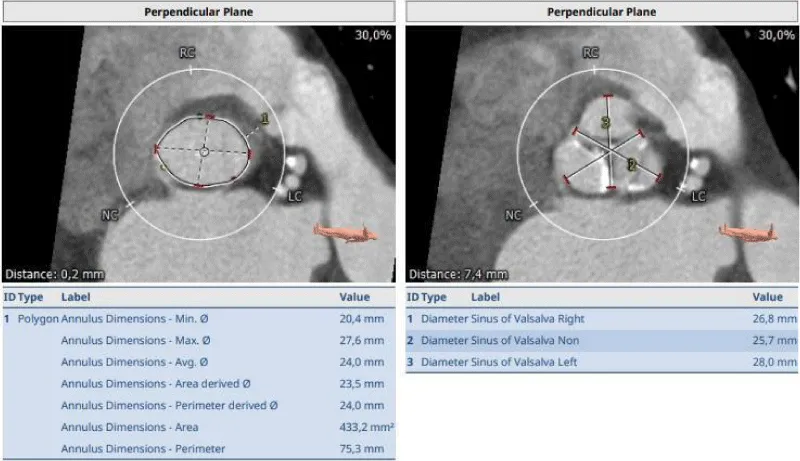
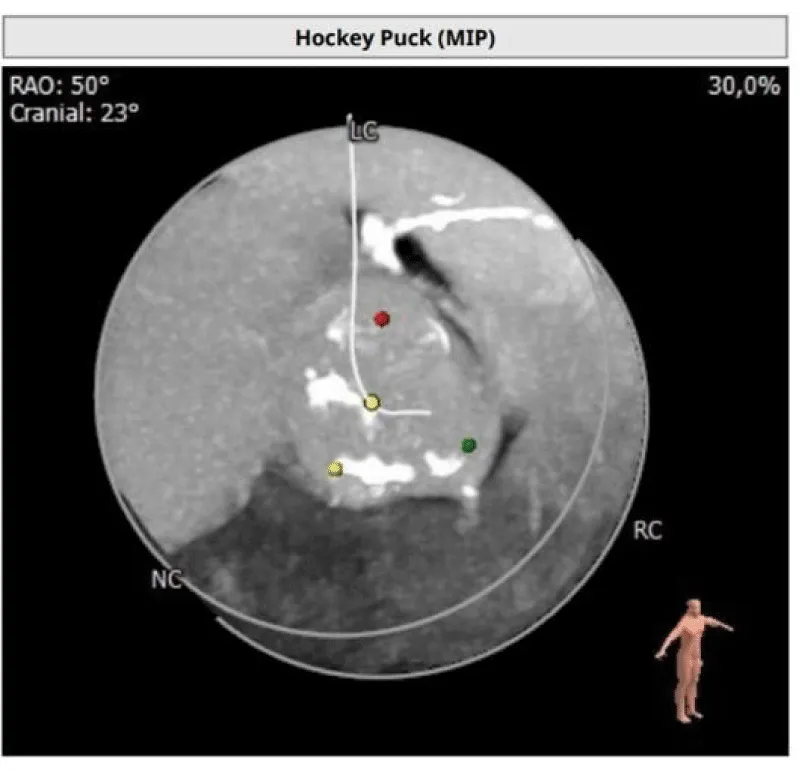
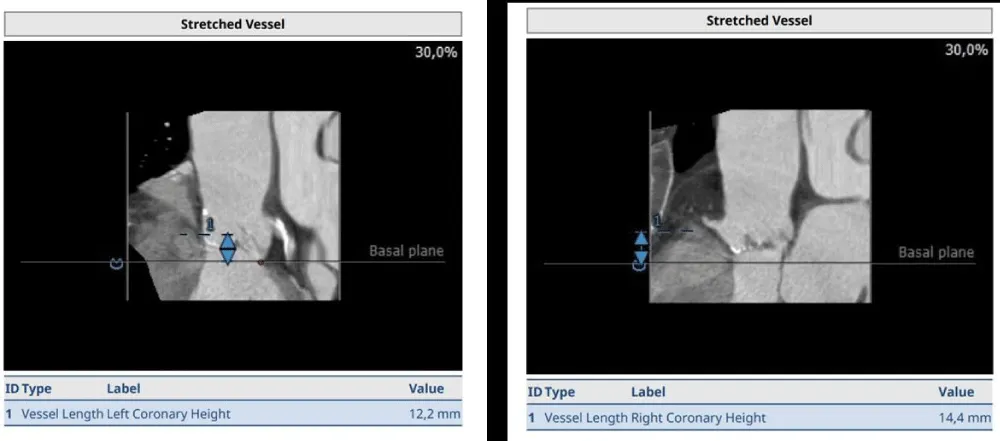
The clinical case concerns an 81-year-old woman with a history of systemic arterial hypertension, hypercholesterolemia, paroxysmal atrial fibrillation, chronic kidney disease, iron-deficiency anemia, and bilateral carotid atheromatosis for which she underwent a Carotid Endarterectomy (CEA) procedure. Additionally, she had a history of breast carcinoma, treated with surgery, subsequent chemotherapy, and ongoing Letrozole therapy. In 2019, the patient presented to the emergency department due to exertional dyspnea and low-threshold angina. During hospitalization, high average ventricular response (109 BPM) atrial fibrillation was diagnosed. Routine lab tests showed rising of hsTN-I (peak 800 pg/dL) and pro-BNP (peak 2476 pg/mL) and anemia Hb 9.1 g/dL. Echocardiography revealed severe depression of the left ventricular function (ejection fraction of 30%, with antero-apical akinesia) and low-flow-low-gradient aortic stenosis (mean gradient of 26 mmHg and AVAi of 0.5 cmq/m2 and SVi 29 mL/m2). So it was performed a Dobutamine stress Echo showed a rise of SVi up to 38 mL/m2 and a mean aortic gradient of up to 48 mmHg. Coronary angiography showed the right dominant coronary artery, of good caliber and course, with no angiographically significant lesions (Figure 1). The Left Main artery, with good caliber, had a 90% calcific stenosis at the distal segment, involving the proximal segment of the Left Anterior Descending artery. The Left Circumflex artery, with good caliber and course, presented a 30% ostial stenosis. The Intermediate Branch, with good caliber and course, showed 80% stenosis at the proximal segment (Figure 2). The Left Anterior Descending artery, also of good caliber and course, was diffusely atherosclerotic with a long, severely calcific 80% stenosis at the mid-distal segment (Figure 3). Therefore, the case was discussed by a multidisciplinary Heart team at our center. The STS score calculated indicated a mortality risk of 11.4%. Due to this high risk, surgical treatment was not considered, and the patient underwent a four-step percutaneous intervention. Before the intervention, a CT angiography was performed, revealing an aortic annulus area of 433.2 mm2, a perimeter of 75.3 mm, and a coronary height from the annulus of 10 mm. The right common femoral artery mean diameter was 6.1 mm2 and the external iliac artery was 8.4 mm2 with moderate tortuosity without significant calcification (Figure 4-6). Therefore, a 23 mm Edwards Sapien 3 valve was chosen. Our strategy consisted of 4 steps:
Figure 1: Right dominant coronary artery, of good caliber and course, with no angiographically significant lesions.
Figure 2: The Left Main artery, with good caliber, had a 90% calcific stenosis at the distal segment, involving the proximal segment of the Left Anterior Descending artery. The Left Circumflex artery, with good caliber and course, presented a 30% ostial stenosis. The Intermediate Branch, with good caliber and course, showed 80% stenosis at the proximal segment.
Figure 3: The Left Anterior Descending artery, also of good caliber and course, was diffusely atherosclerotic with a long, severely calcific 80% stenosis at the mid-distal segment.
Figure 4: Before the intervention, a CT angiography was performed, revealing an aortic annulus area of 433.2 mm2, a perimeter of 75.3 mm, and a coronary height from the annulus of 10 mm. Therefore, a 23 mm Edwards Sapien 3 valve was chosen.
Figure 5: CT angiographic visualization of the vascular access route, displaying the right common femoral artery with a mean diameter of 6.1 mm^2 and the external iliac artery at 8.4 mm^2. Moderate tortuosity without significant calcification is noted, aiding in the strategic planning of vascular access for the percutaneous intervention.
Figure 6: An image detailing the absence of significant calcification in the patient’s vascular access pathway, further elaborating on the favorable anatomical conditions for the percutaneous approach. This image complements the data presented in Figure 5 by highlighting the minimal calcific burden, which facilitates the procedural planning and execution of the transcatheter interventions.
1) Aortic Valvuloplasty
2) Impella LV support
3) Atherectomy and stent implantation on LM and LAD
4) TAVI
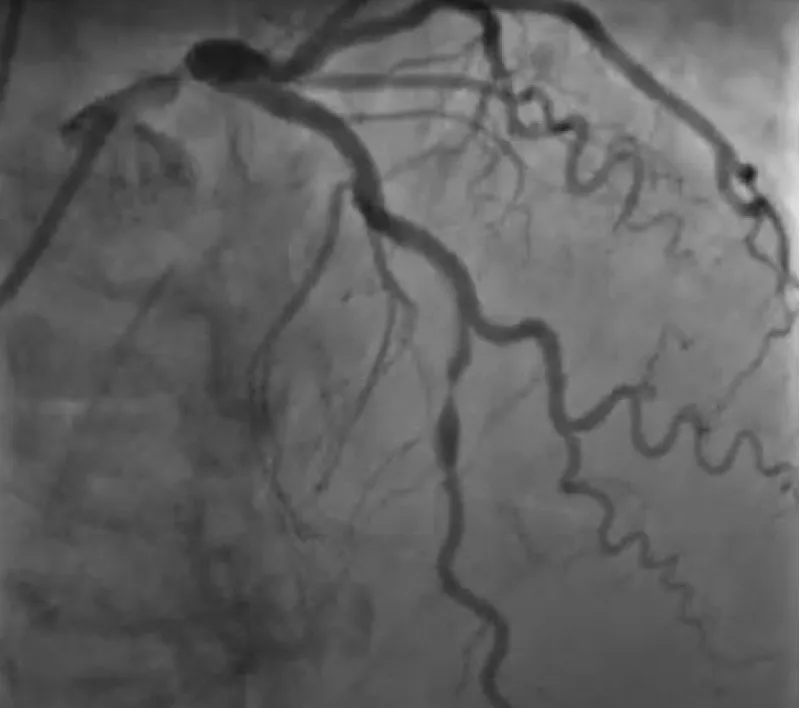
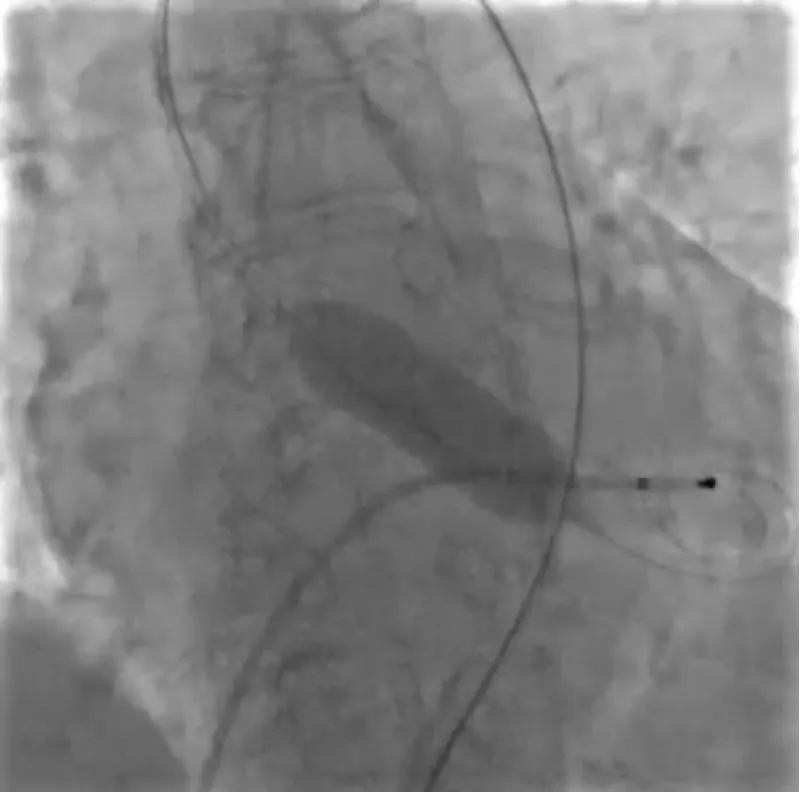
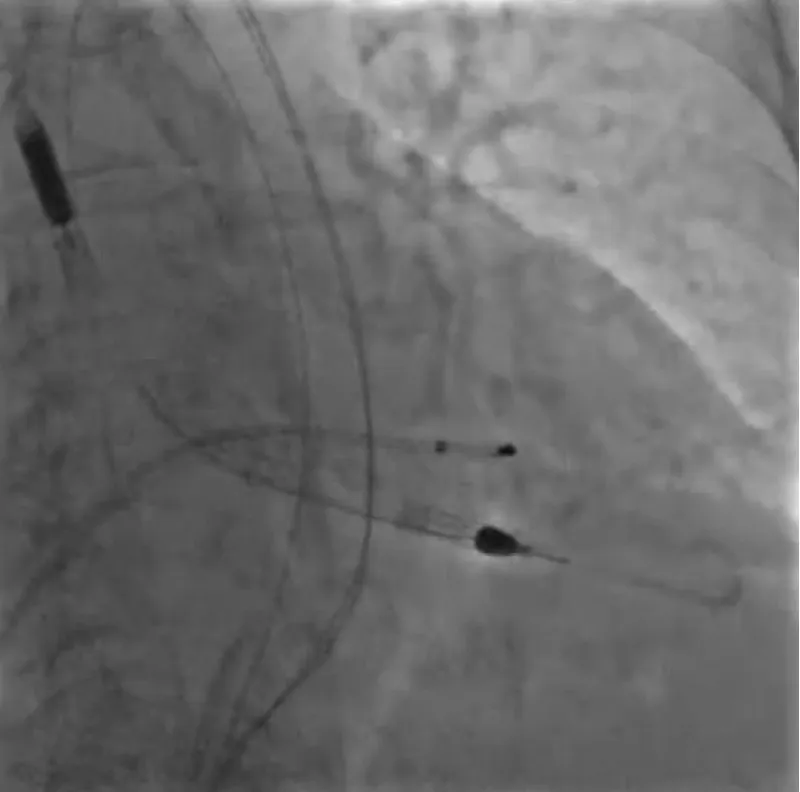
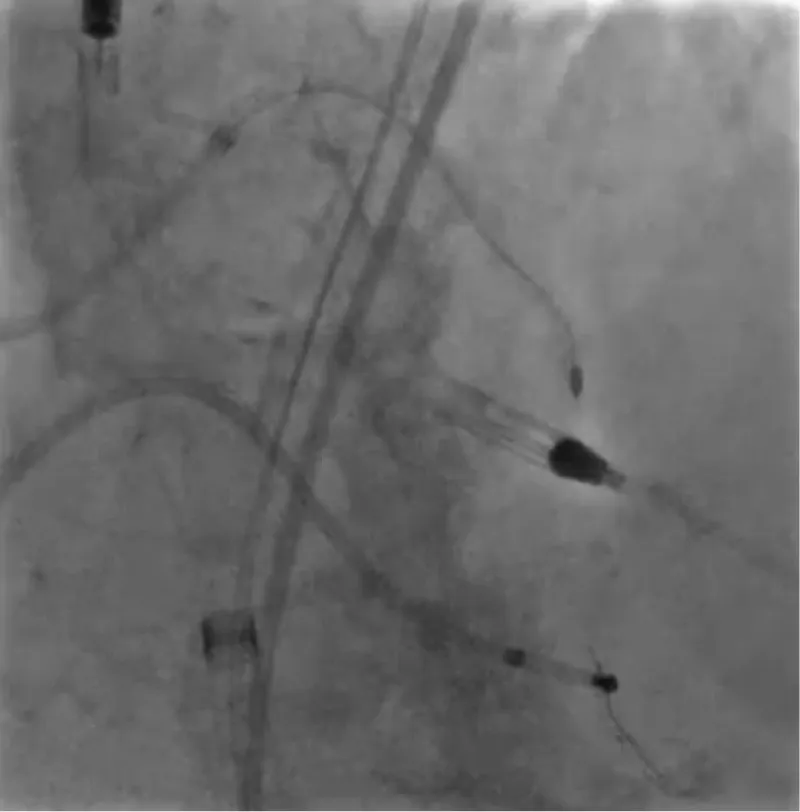
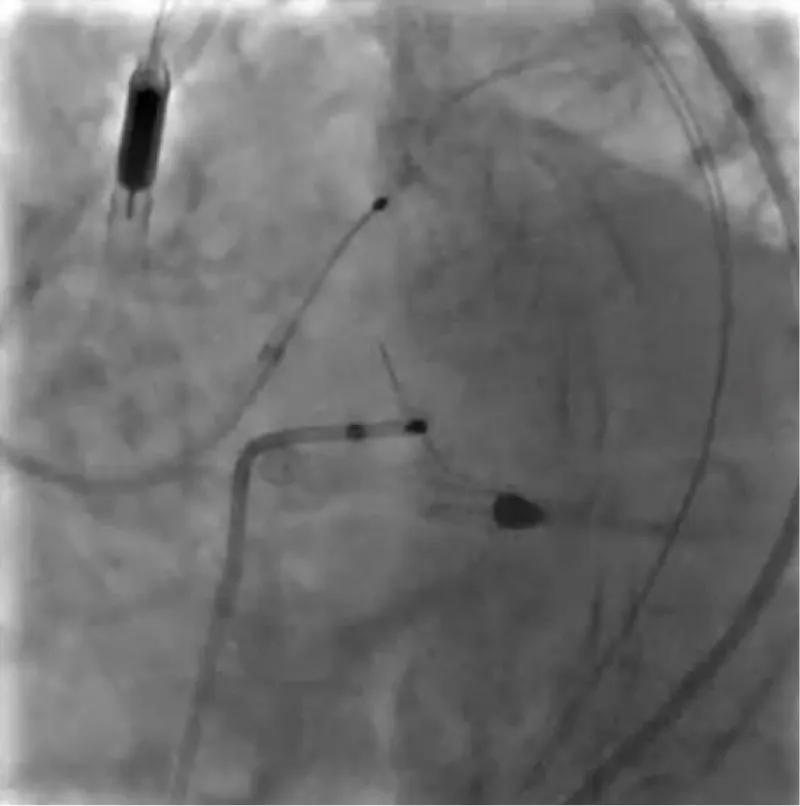
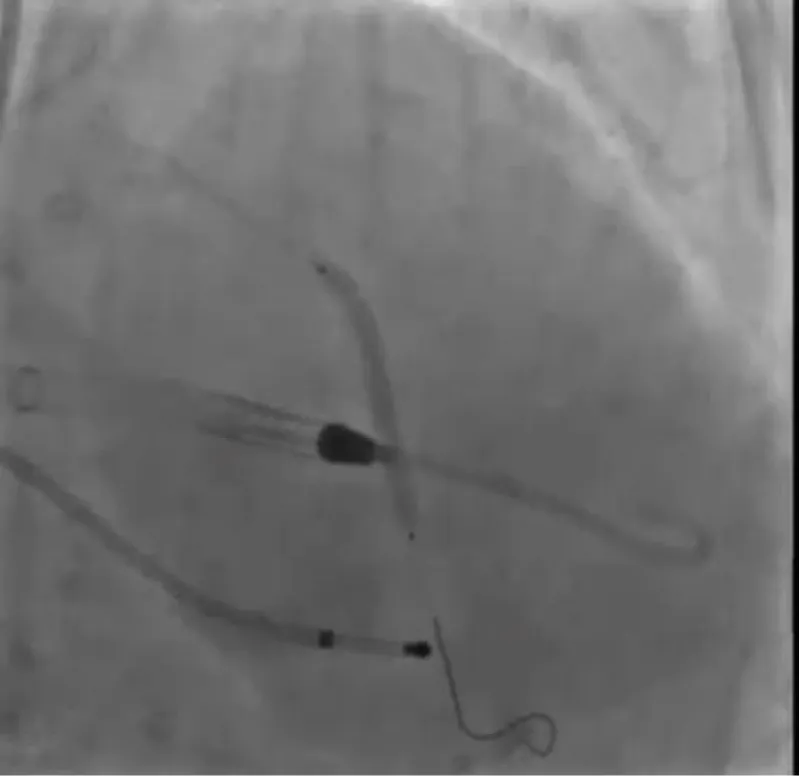
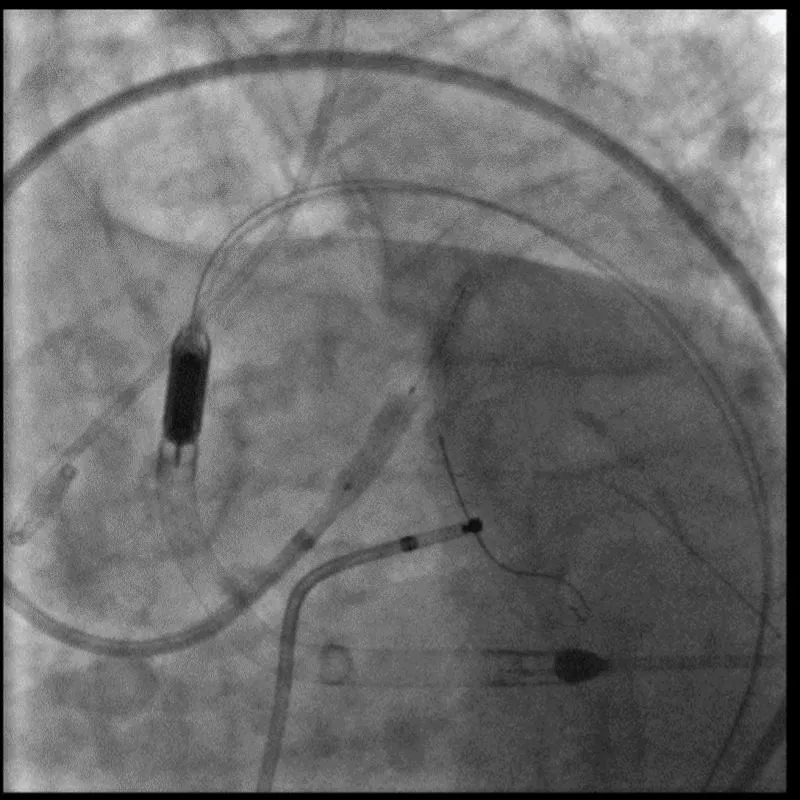
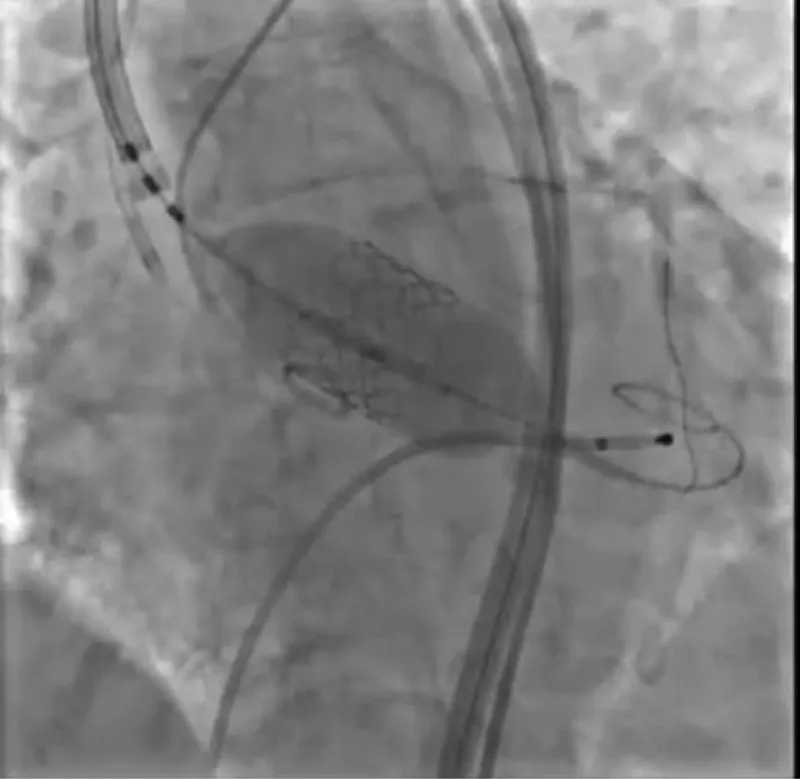
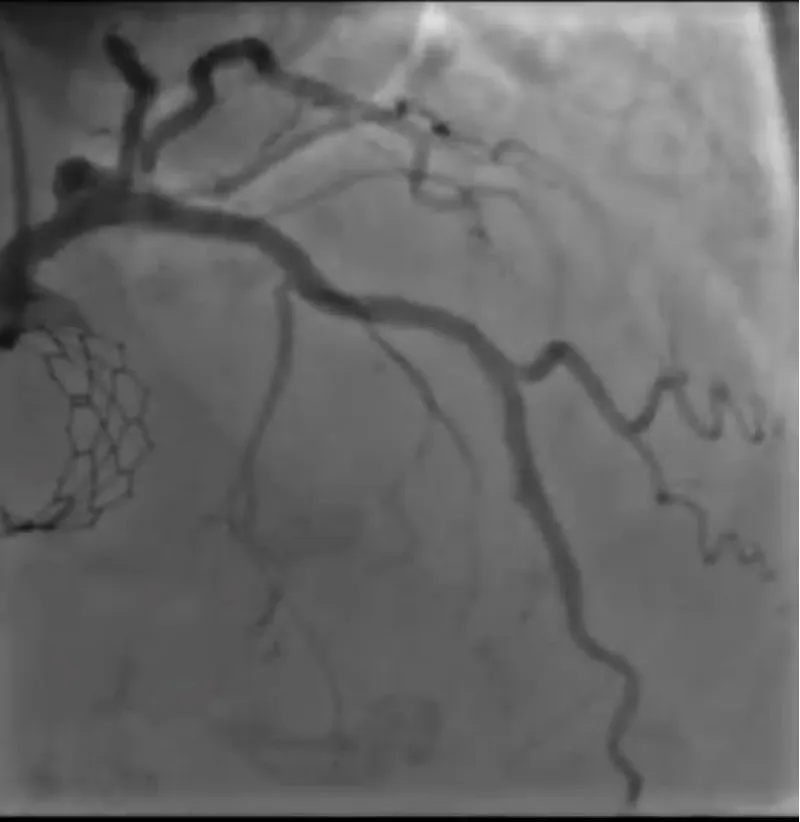
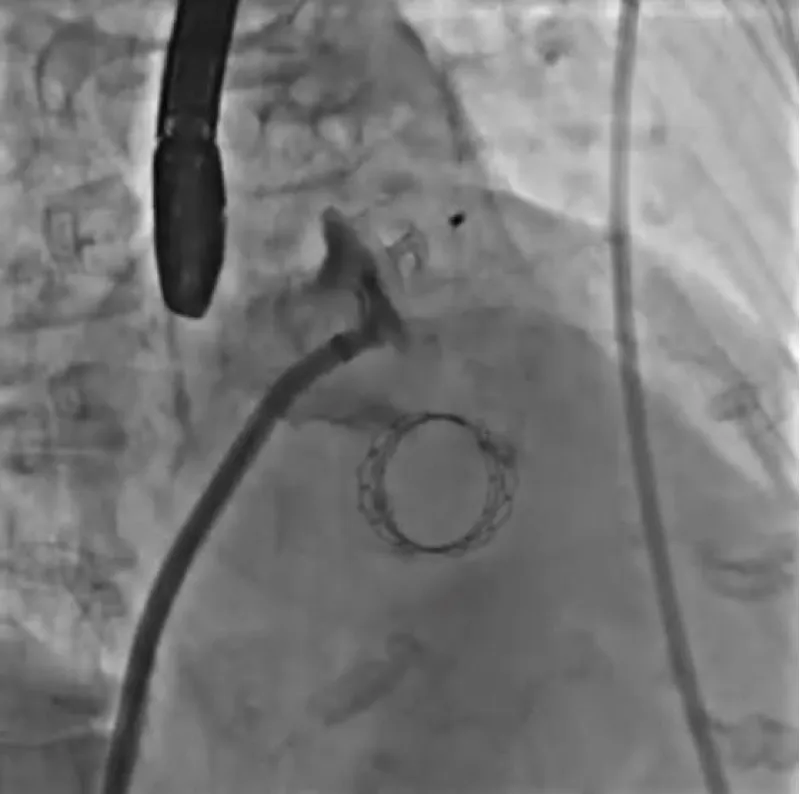
With the support of deep sedation, an angio-guided right femoral access was obtained, and a 12F introducer was advanced. We crossed the aortic valve with an Amplatzer left 1 catheter with a 0.035” wire and exchanged the wire with an extra-stiff Safari. We performed aortic Valvuloplasty with a Nucleus balloon 18 x 40 mm. (Figure 7) Then, we exchanged the wire and advanced an IMPELLA CP device, starting a protected PCI on LM. (Figure 8) An EBU 4.0 catheter was advanced, the stenosis was crossed on the Left Main (LM) and mid-Left Anterior Descending artery (LAD) using a BMW guide wire, and with the aid of a microcatheter a 0.014” Rotawire was positioned, followed by rotational atherectomy using a 1.5 mm burr on LM and mid LAD, (Figure 9) and a 2.0 mm burr on LM alone (Figure 10). This was followed by predilation with a 2.5 x 20 mm SC balloon and the implantation of a 2.5 x 32 mm Synergy drug-eluting stent (Figure 11). Then, the Left Main was predilated with a 3.5 x 12 mm NC balloon, and a 4.0 x 15 mm Xience Sierra drug-eluting stent was implanted and post-dilated with a 4.5 x 8 mm SC balloon (Figure 12). Immediate angiographic control confirmed a successful result. Then we removed the IMPELLA CP device and advanced a 14F e-sheath Edwards on the right femoral artery, crossed the aortic valve with a Safari extra-stiff wire, and performed a Sapien 3 23 mm valve implantation with a good angiographic result (Figure 13). Post-procedural echocardiographic and angiographic assessment confirmed the valve was correctly positioned, with no significant gradient and a minimal residual leak. The procedure was uneventful, and the patient was discharged on the fourth day with triple therapy: Aspirin 100 mg once daily, Clopidogrel 75 mg once daily, and Apixaban 2.5 mg twice daily. At the quarterly follow-up, the patient reported hospitalization for anemia, requiring a transfusion of 3 units of packed red blood cells. Endoscopic exams were negative, and hypochromic, normocytic anemia persisted. Given the patient’s CHA2D2-VASc score of 5, HAS-BLED score of 4, and recent dual Drug-Eluting Stent (DES) implantation, she was admitted for left atrial appendage percutaneous closure. Before this procedure, a repeat coronary angiography showed good results in the previously implanted stents (Figure 14). Subsequently, through a right femoral percutaneous approach using a 12F introducer, a 22 mm Amulet device was positioned for left atrial appendage closure (Figure 15). The procedure was completed without complications. At the 4-year follow-up, the patient presented in good hemodynamic status, with an echocardiographic ejection fraction of 55%. She continued on clopidogrel 75 mg once daily therapy.
Figure 7: Valvuloplasty with balloon Nucleus 18 mm.
Figure 8: Impella CP Device.
Figure 9: Rotational atherectomy with Rotablator Burr 1.5 mm on LDA.
Figure 10: Rotational atherectomy with Rotablator Burr 2.0 mm on LM.
Figure 11: PTCA on LAD.
Figure 12: PTCA on LM.
Figure 13: Sapien 3 23 mm.
Figure 14: Satisfactory long-term performance of the previously implanted stents.
Figure 15: Amplatzer Amulet 22 mm.
The Transcatheter Aortic Valve Replacement (TAVI) has become an established procedure for patients with severe aortic stenosis who are aged over 75 years or present a high risk for surgical aortic valve replacement (SAVR), typically indicated by an STS-PROM score or EUROSCORE higher than 8% [1]. Given the clinical presentation of the patient and the rise and fall of troponins indicating a myocardial infarction, it was decided to perform a PTCA (Percutaneous Transluminal Coronary Angioplasty) [2,3]. Moreover, rotational atherectomy represents a safe and effective approach for patients with complex Coronary Artery Disease (CAD), ensuring lesion preparation, especially in heavily calcified stenoses [4]. In this context, the use of Balloon Aortic Valvuloplasty (BAV), followed by Impella®-assisted Left Main Coronary Artery (LMCA) Percutaneous Coronary Intervention (PCI), is feasible in intermediate and high surgical risk patients with severe aortic stenosis as a bridge to TAVR [5]. The application of Left Ventricular (LV) support through the Impella® device provided adequate hemodynamic stability, enabling the performance of high-risk angioplasty in patients with severe coronary artery disease, thereby minimizing the risk of hemodynamic instability [6]. This integrated approach appears to be safe and technically feasible in patients with severe aortic stenosis before undergoing TAVI [7]. Furthermore, the decision to perform TAVI in the same session leveraged the benefits of reduced risk and enhanced procedural efficiency. This strategy is increasingly recognized as a safe and feasible option, optimizing patient outcomes by minimizing the number of invasive procedures and associated recovery time [8]. The subsequent left atrial appendage closure, executed three months later, was a strategic move to mitigate the patient’s elevated risk of hemorrhage, a crucial consideration due to ongoing anticoagulation therapy. This decision aligns with current best practices in managing bleeding risks in cardiac patients, especially in the context of balancing stroke and bleeding risks, furthermore, LAAO devices have been found to be safe and effective with the most recent data documenting procedural success rates of 97% - 98% [9]. The decision to opt for triple therapy instead of dual antiplatelet therapy was influenced by the existing guidelines at the time of the procedure [10]. These guidelines recommended triple therapy considering the available data at that time and before the release of the Augustus study [11], which subsequently contributed to redefining antithrombotic management practices [12].
In this patient, the execution of high-risk Percutaneous Coronary Intervention (PCI) assisted by the deployment of the Impella device, followed by Transcatheter Aortic Valve Implantation (TAVI), proved to be an effective and safe treatment choice, as evidenced by a follow-up period extending to four years. Furthermore, the subsequent closure of the left atrial appendage allowed for the discontinuation of anticoagulant therapy, a significant step given the patient’s high hemorrhagic risk. This case exemplifies the successful integration of advanced cardiovascular interventions, tailored to address the complex needs of high-risk patients and underscores the importance of a strategic, patient-centric approach in cardiac care.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, Bonis MD, Paulis RD, Delgado V, Freemantle N, Gilard M, Haugaa K, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. ESC/EACTS Scientific Document Group , ESC National Cardiac Societies , 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Heart Journal. 2022; 43 (7): 561-63.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. ESC Scientific Document Group, Fourth universal definition of myocardial infarction (2018). European Heart Journal. 2019; 40 (3):237-269. https://doi.org/10.1093/eurheartj/ehy462
- Neumann FJ, Uva MS, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk, Stuart JH, Peter J, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. ESC Scientific Document Group. 2019; 40 (2): 87-165. https://doi.org/10.1093/eurheartj/ehy394
- Mousa MAA, Bingen BO, Al Amri I, Mertens BJA, Taha S, Tohamy A, Youssef A, Jukema JW, Montero-Cabezas JM. Efficacy and Safety of Intravascular Lithotripsy Versus Rotational Atherectomy in Balloon-Crossable Heavily Calcified Coronary Lesions. Cardiovasc Revasc Med. 2023 Mar;48:1-6. doi: 10.1016/j.carrev.2022.10.011. Epub 2022 Oct 29. PMID: 36336588.
- Diaz Quintero L, Gajo E, Guerrero M, Feldman T, Levisay J. Balloon Aortic Valvuloplasty Followed by Impella®-Assisted Left Main Coronary Artery Percutaneous Coronary Intervention in Patients with Severe Aortic Stenosis as a Bridge to Transcatheter Aortic Valve Replacement. Cardiovasc Revasc Med. 2021 Jan; 22:16-21. doi: 10.1016/j.carrev.2020.06.003. Epub 2020 Jun 3. PMID: 32532627.
- Spiro J, Venugopal V, Raja Y, Ludman PF, Townend JN, Doshi SN. Feasibility and efficacy of the 2.5 L and 3.8 L impella percutaneous left ventricular support device during high-risk, percutaneous coronary intervention in patients with severe aortic stenosis. Catheter Cardiovasc Interv. 2015 May;85(6):981-9. doi: 10.1002/ccd.25355. Epub 2014 Jan 31. PMID: 24408882.
- Kneizeh K, Alnaimi A, Noterdaeme T, Schröder J, Altiok E, Marx N, Almalla M. Protected Percutaneous Coronary Intervention of Unprotected Left Main Using Impella Ventricular Assist Device Before Transcatheter Aortic Valve Implantation: A Single-Center Experience. J Invasive Cardiol. 2022 Mar;34(3):E196-E201. Epub 2022 Jan 20. PMID: 35058376.
- Penkalla A, Pasic M, Drews T, Buz S, Dreysse S, Kukucka M, Mladenow A, Hetzer R, Unbehaun A. Transcatheter aortic valve implantation combined with elective coronary artery stenting: a simultaneous approach†. Eur J Cardiothorac Surg. 2015 Jun;47(6):1083-9. doi: 10.1093/ejcts/ezu339. Epub 2014 Sep 12. PMID: 25217500.
- Holmes DR Jr, Korsholm K, Rodés-Cabau J, Saw J, Berti S, Alkhouli MA. Left atrial appendage occlusion. EuroIntervention. 2023 Feb 6;18(13):e1038-e1065. doi: 10.4244/EIJ-D-22-00627. PMID: 36760206; PMCID: PMC9909459.
- Valgimigli M, Bueno H, Byrne RA, Jean-Philippe C, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN. ESC Scientific Document Group , ESC Committee for Practice Guidelines (CPG) , ESC National Cardiac Societies , 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS), European Heart Journal. 2018; 39 (3): 213-260.
- Lopes RD, Vora AN, Liaw D, Granger CB, Darius H, Goodman SG, Mehran R, Windecker S, Alexander JH. An open-Label, 2 × 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: Rationale and design of the AUGUSTUS trial. Am Heart J. 2018 Jun;200:17-23. doi: 10.1016/j.ahj.2018.03.001. Epub 2018 Mar 9. PMID: 29898844.
- Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH; AUGUSTUS Investigators. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med. 2019 Apr 18;380(16):1509-1524. doi: 10.1056/NEJMoa1817083. Epub 2019 Mar 17. PMID: 30883055.