More Information
Submitted: May 06, 2024 | Approved: May 17, 2024 | Published: May 29, 2024
How to cite this article: Shakya S, Gajurel RM, Poudel CM, Shrestha H, Devkota S, et al. Outcome of Patients Presenting with Peripartum Cardiomyopathy in a Tertiary Care Center of Nepal. J Cardiol Cardiovasc Med. 2024; 9: 081-086.
DOI: 10.29328/journal.jccm.1001183
Copyright License: © 2024 Shakya S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Peripartum cardiomyopathy; Heart failure; Left ventricular systolic dysfunction; Nepal
Abbreviations: ACC: American College of Cardiology; ESC: European Society of Cardiology; LA: Left Atrium; LVEDD: Left Ventricular End Diastolic Dimension; LVEF: Left Ventricular Ejection Fraction; PPCM: Peripartum Cardiomyopathy; ECG: Electrocardiography
Outcome of Patients Presenting with Peripartum Cardiomyopathy in a Tertiary Care Center of Nepal
Smriti Shakya1* , Ratna Mani Gajurel1, Chandra Mani Poudel1, Hemant Shrestha1, Surya Devkota1, Sanjeev Thapa1, Bhawani Manandhar1, Rajaram Khanal1, Suraj Shrestha2 and Manju Sharma1
, Ratna Mani Gajurel1, Chandra Mani Poudel1, Hemant Shrestha1, Surya Devkota1, Sanjeev Thapa1, Bhawani Manandhar1, Rajaram Khanal1, Suraj Shrestha2 and Manju Sharma1
1Department of Cardiology, Manmohan Cardiothoracic Vascular and Transplant Center (MCVTC), Institute of Medicine, Kathmandu, Nepal
2Maharajgunj Medical Campus, Institute of Medicine, Kathmandu, Nepal
*Address for Correspondence: Dr. Smriti Shakya, Department of Cardiology, Manmohan Cardiothoracic Vascular and Transplant Center (MCVTC), Institute of Medicine, Kathmandu, Nepal. Email: [email protected]
Purpose: Peripartum cardiomyopathy is a rare life-threatening cardiomyopathy of unknown etiology with significant maternal morbidity and mortality. It causes heart failure due to left ventricular systolic dysfunction with or without left ventricular dilatation in the last month of pregnancy up to 5 months postpartum in previously healthy women. We aimed to determine short-term outcomes of peripartum cardiomyopathy clinically as well as in terms of left ventricular systolic function and to study the clinical profile and associated risk factors.
Patients and methods: A prospective observational study was carried out in the Department of Cardiology of Manmohan Cardiothoracic Vascular and Transplant Center, Institute of Medicine, Kathmandu, Nepal, from July 2018 to January 2022. All the patients with peripartum cardiomyopathy who presented to the department of cardiology were enrolled in the study and re-evaluated with echocardiography at 6 months.
Results: A total of 68 women met the inclusion criteria. The mean age was 28.38 ± 5.5 years (range 19 to 44 years). The most common clinical presentation was dyspnea followed by lower limb edema. Six (8.8%) patients presented during the last month of pregnancy whereas 62 (91.2%) patients presented in the postpartum period. The mean left ventricular ejection fraction on presentation was 30.01 ± 8.54. A full recovery was observed among 60.29% at 6 months. The mortality rate was 4.41%.
Conclusion: Timely diagnosis and management of peripartum cardiomyopathy with standard therapy for heart failure leads to better recovery of left ventricular systolic function.
Peripartum cardiomyopathy (PPCM) is a rare life-threatening cardiomyopathy of unknown etiology that occurs in the peripartum period in previously healthy women. It is a diagnosis of exclusion though multiple mechanisms have been considered [1]. The European Society of Cardiology’s (ESC) working group on PPCM defined it as cardiomyopathy with a decreased left ventricular ejection fraction (LVEF usually 45%), manifesting as heart failure toward the end of pregnancy or in the months after delivery, typically in the last month of pregnancy and up to 5 months postpartum in a woman without structural heart disease [1,2]. It has a rapid clinical course with a high possibility for complete recovery but has a tendency to revert in succeeding pregnancies or even surviving with heart failure or mortality [3].
The incidence of PPCM is highly variable with geographic variation. The incidence in the United States ranges from 1 in 3000 to 4000 live births [3]. The incidence in Africa and Asia is around 1 in 1000 live births. But certain areas such as Haiti show an incidence of 1 in 300 live births and in Nigeria, the incidence has been reported as high as 1 in 100 live births [4]. The etiology of PPCM though not fully understood, few suggested pathophysiological mechanisms are nutritional deficiencies, viral myocarditis and autoimmune processes, and prolactin, 16 kDa prolactin, and Cathepsin D hypothesis [5–8]. The associated risk factors include African ancestry, older maternal age, multi-gestational pregnancy, multigravida or parity, pre-eclampsia, hypertension, use of tocolysis, and low socioeconomic condition [9-11].
In this study, we aimed to determine short-term outcomes of peripartum cardiomyopathy clinically as well as in terms of left ventricular systolic function and to study the clinical profile and associated risk factors.
Study design
A prospective observational study was carried out in the Department of Cardiology, Manmohan Cardiothoracic Vascular and Transplant Center, Institute of Medicine, Kathmandu, Nepal, from July 2018 to January 2022.
Study population
Patients with peripartum cardiomyopathy fulfilling pre-defined inclusion criteria who presented to the Department of Cardiology were enrolled in the study.
Sample size
The inclusion criteria included the patients with LV ejection fraction of less than 45% with or without left ventricular dilatation with no other obvious cause of LV dysfunction in the last month of pregnancy or 5 months postpartum. The preexisting LV dysfunction before or early during pregnancy, LV dysfunction due to thyrotoxicosis, valvular heart disease, coronary artery disease, alcohol abuse, or any other likely attributable causes were excluded. In addition, patients with inadequate data of interest, history of COVID-19 myocarditis, or active COVID-19 infection at the time of enrollment in the study were excluded. All the eligible patients during the study period (July 2018 to January 2022) were considered and a total of 68 women with peripartum cardiomyopathy were included in this study.
Data collection and statistical analysis
The demographic and clinical parameters were recorded along with twelve-lead electrocardiography (ECG) and transthoracic echocardiography. LVEF was calculated by M-mode or Simpson’s method at the time of enrollment. The left ventricular end-diastolic dimension (LVEDD) was taken in parasternal long-axis view. Other cardiac chambers were also evaluated. The LA dimension of more than 40 mm was considered to be dilated. The LV function was considered normal if above 50%, mild dysfunction if 40% to 49%, moderate dysfunction if 30% to 39%, and severe LV systolic dysfunction if LVEF was less than 30% according to the American College of Cardiology (ACC). The LV is said to be dilated if LVEDD is > 52 mm. The echocardiography findings repeated at 3 months and subsequently at 6 months to evaluate the LV systolic function were also evaluated. Early recovery is defined as the recovery that occurs within 6 months. An LVEF of greater than 50% was considered to indicate complete recovery, while an ejection fraction of less than 50% indicated recovery with a decreased ejection fraction. All patients received the guideline-recommended evidence-based standard medical treatment for heart failure which was appropriate in antepartum and postpartum settings. Statistical Package for Social Sciences version 21.0 was used for statistical analysis. Standard descriptive statistics were used to describe the data. Categorical variables were reported as frequency (percentages) whereas the continuous variables were reported as mean ± standard deviation. Multivariate regression analysis was performed to identify independent patient factors that were associated with recovery, along with their adjusted Odds ratios (aOR). P < 0.05 was considered to be statistically significant.
Ethical approval
The ethical approval was obtained from the Institutional Review Committee of the hospital with an ethical approval number of 12(6-11)E2/77/78 prior to the commencement of the study and has been conducted in accordance with the declaration of Helsinki.
A total of 77 women with peripartum cardiomyopathy were identified during the study period. Nine women were excluded from the study as two had co-existing hyperthyroidism (due to concern of causing bias for LV dysfunction), four did not come for follow-up after discharge, and three patients were doubtful of their pre-existing left ventricular dysfunction. A total of 68 women met the inclusion criteria. Demographic and basic characteristics are shown in (Table 1). The mean age was 28.38 ± 5.5 years (range 19 to 44 years).
| Table 1: Baseline characteristics of PPCM patients. | |
| Baseline characteristics | |
| Mean Age (years) | 28.38 ± 5.5 |
| Primipara | 48(70.59%) |
| Multipara | 20(29.41%) |
| Multiple pregnancies | 3(4.41%) |
| Preeclampsia | 4(5.88%) |
| Pregnancy induced HTN | 11(16.18%) |
| Diabetes mellitus | 4(5.88%) |
| Past history of PPCM | 2(2.94%) |
| Mean parity | 1.39 ± 0.81 |
Dyspnea and lower limb swelling were the most common clinical. Few patients had additional clinical features given in (Table 2). Six (8.8%) patients presented during the last month of pregnancy whereas 62 (91.2%) patients presented in the postpartum period. The mean day at presentation is 23.74 days (range 1 to 90 days).
| Table 2: Presenting symptoms of PPCM patients. | |
| Symptoms | No. of patients (%) |
| Dyspnea | 68(100%) |
| Orthopnea | 40(58.8%) |
| Paroxysmal nocturnal dyspnea | 24(35.3%) |
| Lower Limbs swelling | 42(61.8%) |
| Palpitation | 22(32.4%) |
| Hemoptysis | 4(5.9%) |
| Cardiogenic shock | 5(7.4%) |
| Thromboembolism | 14(20.6%) |
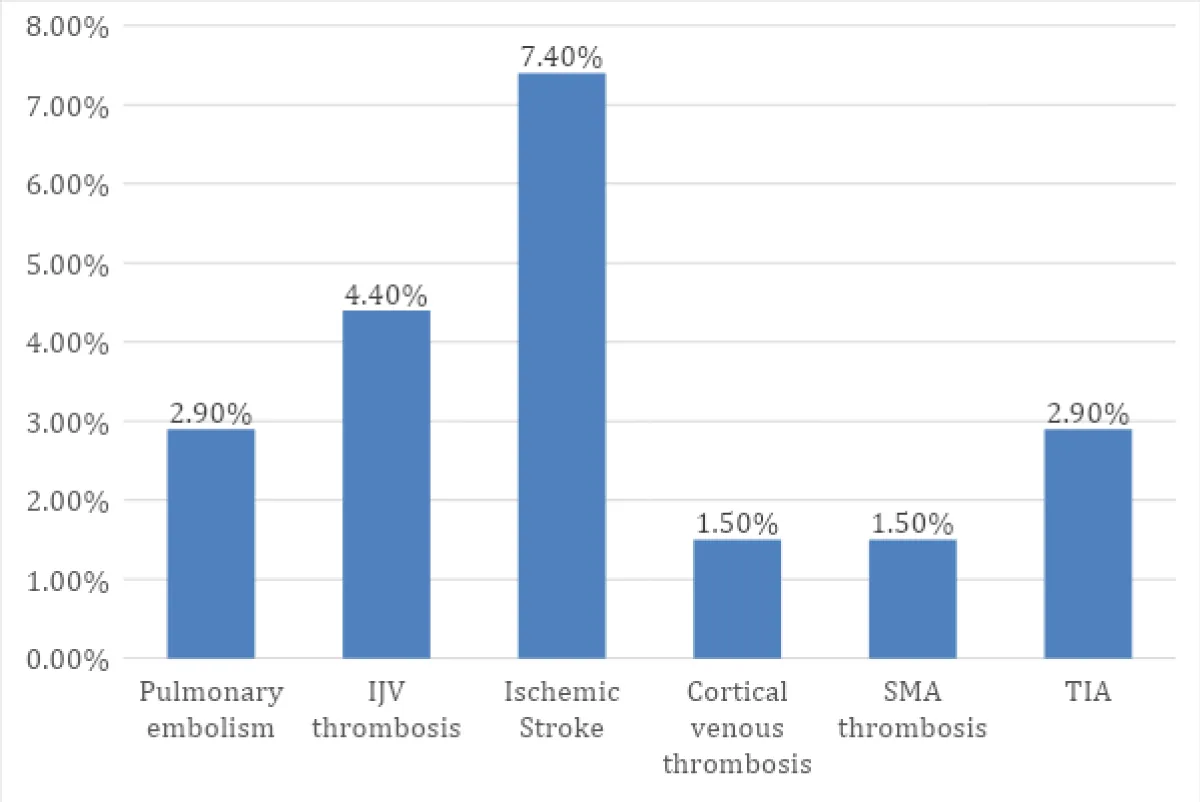
Among 14 patients (20.6%) who had thromboembolism, thrombosis was seen in arteries or veins. There were embolic events such as pulmonary embolism in 2 patients (2.9%), ischemic stroke in 5 patients (7.4%), and transient ischemic attack in 2 patients (2.9%). There was one patient (1.5%) with cortical venous thrombosis (right transverse, sigmoid sinuses), one (1.5%) with SMA thrombosis (mesenteric ischemia), right profunda femoris artery thrombosis, bilateral popliteal artery thrombosis, bilateral renal infarct and three (4.4%) with internal jugular vein thrombosis (Figure 1).
Figure 1: Thrombosis and embolic phenomenon in PPCM patients.
The mean left ventricular ejection fraction on presentation was 30.01 ± 8.54, at 3 months it was 42.38 ± 10.19 and at 6 months it was 50.28 ± 11.31. The majority of patients had severe LV systolic dysfunction i.e. 41 (60.29%) at presentation. At 3 months, the majority had moderate LV systolic dysfunction i.e. 21 (32.30%) and at 6 months, the majority had recovered left ventricular ejection fraction (Table 3). Only 29 patients (42.65%) patients had dilated left ventricle and 25 patients (36.76%) had dilated left atrium on presentation. The left ventricular clot was present in 6 patients (8.82%).
| Table 3: Left ventricular systolic function of PPCM patients. | |||
| LV systolic function | On presentation | 3 months | 6 months |
| Severe LVSD | 41(60.29%) | 6(9.23%) | 3(4.62%) |
| Mod LVSD | 23(33.82%) | 21(32.30%) | 5(7.69%) |
| Mild LVSD | 4(5.89%) | 14(21.55%) | 16(26.15%) |
| Recovered | 24(35.29%) | 41(60.29%) | |
| Mean LVEF (%) | 30.01±8.54 | 42.38±10.19 | 50.28 ± 11.31 |
The majority of patients had normal vaginal delivery i.e. 42 (61.8%), 23 patients (33.8%) had a cesarean section which was for obstetric causes, 2 patients (2.9%) had forceps delivery and 1 patient (1.5%) had vacuum delivery. There was a stillbirth of 3 patients and intrauterine fetal death of 2 patients. The mortality rate was 3 (4.41%). Two patients died during hospital admission due to cardiogenic shock and intractable heart failure and 1 patient died within 2 weeks of discharge from hospital. All of the three had severe LV systolic dysfunction at baseline. The full recovery was seen in 60.29% at 6 months.
Upon multivariate regression analysis, the significant independent predictors of recovery were found to be age [aOR (95% CI): 1.16 (1.02 – 1.32, p = 0.02), presence of hypertension [aOR (95% CI): 0.20 (0.05 – 0.80), p = 0.02] and presence of LV dilatation [aOR (95% CI): 0.25 (0.07 – 0.90), p = 0.03). Baseline LVEF and multiparity were not found to be independent predictors of recovery. The presence of hypertension and LV dilatation was associated with decreased odds of recovery, a decrease of 80% and 75% respectively compared to those who did not have hypertension and LV dilatation.
Among 68 patients, all of them received diuretics initially, 52 (76.47%) patients received ACEI/ARB, 54 (79.41%) patients received beta-blockers, 6 (8.82%) patients received digoxin, 4 (5.89%) patients received bromocriptine (only in those who had a stillbirth and intrauterine fetal death), 14 (20.6%) patients received oral anticoagulant, 11 (16.18%) patients received inotropes and 4 (5.88%) patients were kept under mechanical ventilation. About 7 (10.29%) patients left medications after the initial 3 months of follow-up, mostly due to the inaccessibility of medicines/financial constraints in the rural area where they reside. Among those who left medications, 5 patients (71.43%) had good recovery.
Peripartum cardiomyopathy is a form of idiopathic cardiomyopathy associated with the pregnancy state. In this study, we studied the clinical and echocardiographic profile of PPCM patients and followed the patients at 3 months and 6 months to observe the recovery rate in left ventricular systolic function with standard heart failure therapy.
Compared to the antepartum or peripartum periods, the postpartum period exhibited the majority of PPCM diagnoses. This can be the result of underreporting or a failure to diagnose PPCM related to a few symptoms previously. However similar findings were observed in other studies as well [12]. The identified risk factors in the Western population are twin gestation, multiparity, advanced maternal age, and preeclampsia [13,14]. In our study the mean maternal age was 28.38 ± 5.5 years (range 19 to 44 years) and the majority of them were young primigravidae. Although PPCM appears to be more common in older women with high parity, however, it’s crucial to bear in mind that 24% - 37% of cases may occur in young primigravid patients [8,14–16].
PPCM in the South Asian population is seen among younger age groups. This could be explained by an earlier age at the time of marriage and subsequent pregnancy [17]. The mean parity was 1.39 ± 0.81 where multiparity used to be the traditional risk factor for PPCM [18]. There were only 3 (4.41%) patients with twin pregnancies in this study. Preeclampsia was seen in 4 (5.88%) and pregnancy-induced hypertension in 11 (16.18%) unlike previous studies where hypertension was the most common comorbidity [19].
The common clinical presentations observed were dyspnea and peripheral edema in our study. Twelve (17.6%) had thromboembolic events. Few cases have been reported where patients with PPCM presented with thromboembolic events as the initial manifestation [20,21]. However, there was no mortality in the patients with documented thromboembolic events, unlike a study where it directly caused death in patients with PPCM within 3 months of follow-up [22]. As a result of the associated hypercoagulable state, venous stasis, and diffuse vascular injury, PPCM is one of the established clinical causes of thrombo-embolic phenomena in young women [23]. In the Peripartum Cardiomyopathy in Nigeria (PEACE) registry, an intra-cardiac thrombus on echocardiography was found in about 6.4% of PPCM patients [24]. In our study, the left ventricular clot was present in 8.82% of patients. The LV thrombus has been found on initial echocardiography in 10% to 17% of patients in a study. Several reports have shown severe thromboembolic events, including embolization to the coronary, pulmonary, peripheral, and cerebral arteries in this condition [11]. Numerous factors, including the hypercoagulable state of pregnancy, LV dilatation and dysfunction, endothelial damage, venous stasis, and prolonged bed rest following instrumental births or cesarean sections, may contribute to the increased incidence of thromboembolism [25]. The mean left ventricular ejection fraction on presentation was 30.01% ± 8.54%. The majority of patients had severe LV systolic dysfunction (60.29%) at presentation. Only 42.65% of patients had dilated left ventricles at presentation.
The outcomes are variable, women may have a complete recovery, persistent myocardial dysfunction, HF, or rapid deterioration [5]. In our study, the recovery in left ventricular systolic function was 35.29% in 3 months and 60.29% in 6 months of follow-up. The recovery rates are variable in different studies. A multicenter study conducted in the United States showed that 71% of the women recovered LVEF to > 50%, and only 13% experienced severe episodes or chronic cardiomyopathy with EF < 35% in one year [26]. In many of the African studies, only 21% to 46% of the patients had complete recovery of LV function at six months of follow-up and the majority of patients did not achieve complete recovery of LV function [22,27,28]. In a study done in India, about 61% of patients had full LV functional recovery at the end of 6 months after PPCM diagnosis [29]. 36% had complete recovery of LVEF at 3 months in a study done in the eastern part of Nepal [30]. The earlier studies suggested that recovery frequently occurs within the first 6 months [10,11] but studies with longer follow-up found that delayed recovery is possible, even several years after diagnosis [14,27,31,32]. Among the study patients, 2 (2.94%) had a past history of peripartum cardiomyopathy, one of whom recovered and the other one had persistent LV systolic dysfunction at 6 months. The exact mechanism behind myocardial recovery in PPCM is not fully understood, and factors influencing myocardial recovery in PPCM remain an active area of research. However, several hypotheses have been proposed. Some researchers believe that elevated levels of prolactin during pregnancy may contribute to the development of PPCM, which decreases after delivery and might facilitate myocardial recovery [33]. Another theory suggests that an imbalance in angiogenic factors, such as vascular endothelial growth factor (VEGF) and soluble fms-like tyrosine kinase-1 (sFlt-1), may play a role in the pathogenesis of PPCM. Restoration of this balance postpartum may promote myocardial recovery. Some believe that the heart has intrinsic mechanisms for repair and remodeling that might be activated post-delivery, leading to the improvement of myocardial function. Genetic factors and treatments with medications might contribute to myocardial recovery in some patients. It is important to note that while some women with PPCM experience complete recovery of myocardial function, others may have persistent cardiac dysfunction or even progress to end-stage heart failure requiring advanced therapies [34–36].
The mortality rate in our study was 4.41%. The reported mortality rates linked to PPCM in the US have ranged widely between 0% and 19%. Significant discrepancies in the reports are likely caused by different patient groups, therapies, and possible reporting bias [25]. Regarding the timing of the mortality, Goland, et al. revealed that most of them died either suddenly (38%) or due to progressive heart failure (45%), 18% of deaths occurred within one week, and 87% within 6 months of diagnosis [37]. Women with baseline LVEF ≤ 25% and those whose diagnosis of PPCM was delayed, were shown to have greater mortality rates. Studies on PPCM found that advanced mother age, higher parity, postpartum timing, and a history of hypertension were predictors of worse outcomes. About 61% of patients had full LV functional recovery at 6 months following PPCM diagnosis, with a 4.7% death rate in a few studies [9,29], whereas in another study of India mortality was 6% [17].
In this study, we found that the presence of hypertension and LV dilatation decreased the chances of recovery. A study by Goland, et al. found LVEF > 30% and LV end-diastolic dimension < 55 mm to be significantly related to LV recovery among patients with PPCM, suggesting a relationship between the severity of initial myocardial insult and recovery [38]. However, although the LVEDD < 55 mm showed better recovery, the baseline LVEF at presentation and multiparity were not found to be independent predictors of recovery.
In our study, most of the patients received guidelines directed to standard heart failure therapy (ACEI/ ARBs and beta-blockers). The difference in choice of heart failure medication depended on underlying patients’ comorbidities and drug contraindications, e.g., no ACEIs/ARBs for those who developed renal impairment and had hyperkalemia, no beta-blockers for hypotension or bradycardia, digoxin for those patients who couldn’t tolerate beta-blockers, and bromocriptine to patients with stillbirth and intrauterine fetal death as it couldn’t be used for breastfeeding mothers. The oral anticoagulant was used in only those patients with thromboembolic phenomenon. About 10.29% left medications after the initial 3 months of follow-up. Among those who left medications, the majority had a good recovery.
It’s unclear how standard heart failure treatment and recovery are related. The rate of recovery before the advent of contemporary heart failure therapy was comparable to rates reported in recent trials using optimal heart failure treatment [39,40], and early recovery frequently occurred prior to the up-titration of medications to optimal therapeutic levels [11,25]. However, early initiation of optimal anti-failure therapy and strong adherence leads to favorable clinical outcomes [28].
Limitations
There are a few limitations to this study. This was a single-center study with a small sample population and short-term follow-up that may affect the generalizability and validity of this study. Long-term follow-up is imperative to cast more light on the natural history of treated PPCM patients.
Peripartum cardiomyopathy is a rare but lethal disease about which little is known. In the Nepalese population, PPCM was common in young primigravidae with most of the recovery occurring in 6 months post-delivery. The presence of hypertension and LV dilation decreased the chance of recovery and those with severe decline in LVEF had greater mortality. Timely diagnosis and early management with standard therapy for heart failure are utmost for favorable outcomes.
Consent: Written informed consent was taken from all the patients/patients’ parties before the start of this study.
- Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, Ansari A, Baughman KL. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000 Mar 1;283(9):1183-8. doi: 10.1001/jama.283.9.1183. PMID: 10703781.
- European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM); Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L; ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011 Dec;32(24):3147-97. doi: 10.1093/eurheartj/ehr218. Epub 2011 Aug 26. PMID: 21873418.
- Bhattacharyya A, Basra SS, Sen P, Kar B. Peripartum cardiomyopathy: a review. Tex Heart Inst J. 2012;39(1):8-16. PMID: 22412221; PMCID: PMC3298938.
- Arany Z, Elkayam U. Peripartum Cardiomyopathy. Circulation. 2016 Apr 5;133(14):1397-409. doi: 10.1161/CIRCULATIONAHA.115.020491. PMID: 27045128.
- Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Jan 21;75(2):207-221. doi: 10.1016/j.jacc.2019.11.014. PMID: 31948651.
- Fett JD, Ansari AA, Sundstrom JB, Combs GF. Peripartum cardiomyopathy: a selenium disconnection and an autoimmune connection. Int J Cardiol. 2002 Dec;86(2-3):311-6. doi: 10.1016/s0167-5273(02)00359-5. PMID: 12419571.
- Lamparter S, Pankuweit S, Maisch B. Clinical and immunologic characteristics in peripartum cardiomyopathy. Int J Cardiol. 2007 May 16;118(1):14-20. doi: 10.1016/j.ijcard.2006.04.090. Epub 2006 Aug 10. PMID: 16904777.
- Sliwa K, Förster O, Libhaber E, Fett JD, Sundstrom JB, Hilfiker-Kleiner D, Ansari AA. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006 Feb;27(4):441-6. doi: 10.1093/eurheartj/ehi481. Epub 2005 Sep 5. PMID: 16143707.
- Karaye KM, Henein MY. Peripartum cardiomyopathy: a review article. Int J Cardiol. 2013;164(1):33-38. doi:10.1016/j.ijcard.2011.11.069
- Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, Green MS, Gollob MH, Haddad H, Birnie DH. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006 Jun 15;97(12):1765-8. doi: 10.1016/j.amjcard.2006.01.039. Epub 2006 Apr 21. PMID: 16765131.
- Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006 Sep;152(3):509-13. doi: 10.1016/j.ahj.2006.02.008. PMID: 16923422.
- Krishnamoorthy P, Garg J, Palaniswamy C, Pandey A, Ahmad H, Frishman WH, Lanier G. Epidemiology and outcomes of peripartum cardiomyopathy in the United States: findings from the Nationwide Inpatient Sample. J Cardiovasc Med (Hagerstown). 2016 Oct;17(10):756-61. doi: 10.2459/JCM.0000000000000222. PMID: 25943626.
- Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli TM. Peripartum cardiomyopathy: a comprehensive review. Int J Cardiol. 2007; 118(3):295-303. doi:10.1016/j.ijcard.2006.08.005
- Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005 Dec;80(12):1602-6. doi: 10.4065/80.12.1602. PMID: 16342653.
- Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005; 111(16):2050-2055. doi:10.1161/01.CIR.0000162478.36652.7E
- Desai D, Moodley J, Naidoo D. Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Trop Doct. 1995; 25(3):118-123. doi:10.1177/004947559502500310
- Prasad GS, Bhupali A, Prasad S, Patil AN, Deka Y. Peripartum cardiomyopathy - case series. Indian Heart J. 2014; 66(2):223-226. doi:10.1016/j.ihj.2014.02.007
- Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006 Aug 19;368(9536):687-93. doi: 10.1016/S0140-6736(06)69253-2. PMID: 16920474.
- Mahowald MK, Basu N, Subramaniam L, Scott R, Davis MB. Long-term outcomes in Peripartum Cardiomyopathy. Open Cardiovasc Med J. 2019;13(1):13-23. doi:10.2174/1874192401913010013
- Carlson KM, Browning JE, Eggleston MK, Gherman RB. Peripartum cardiomyopathy presenting as lower extremity arterial thromboembolism. A case report. J Reprod Med. 2000 Apr;45(4):351-3. PMID: 10804495.
- Twomley KM, Wells GL. Peripartum cardiomyopathy: a current review. J Pregnancy. 2010; 2010:149127. doi:10.1155/2010/149127
- Gambahaya ET, Hakim J, Kao D, Munyandu N, Matenga J. Peripartum cardiomyopathy among cardiovascular patients referred for echocardiography at Parirenyatwa Teaching Hospital, Harare, Zimbabwe. Cardiovasc J Afr. 2017 Jan/Feb;28(1):8-13. doi: 10.5830/CVJA-2016-043. PMID: 28262909; PMCID: PMC5423423.
- Greer IA. CLINICAL PRACTICE. Pregnancy Complicated by Venous Thrombosis. N Engl J Med. 2015 Aug 6;373(6):540-7. doi: 10.1056/NEJMcp1407434. PMID: 26244307.
- Karaye KM, Ishaq NA, Sa'idu H, Balarabe SA, Talle MA, Isa MS, Adamu UG, Umar H, Okolie HI, Shehu MN, Mohammed IY, Sanni B, Ogah OS, Oboirien I, Umuerri EM, Mankwe AC, Shidali VY, Njoku P, Dodiyi-Manuel S, Shogade TT, Olunuga T, Ojji D, Josephs V, Mbakwem AC, Tukur J, Isezuo SA; PEACE Registry Investigators. Incidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: results from the PEACE Registry. ESC Heart Fail. 2020 Feb;7(1):235-243. doi: 10.1002/ehf2.12562. Epub 2020 Jan 28. PMID: 31990449; PMCID: PMC7083508.
- Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011; 58(7):659-670. doi:10.1016/j.jacc.2011.03.047
- McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J 3rd, Wu WC, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD; IPAC Investigators. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015 Aug 25;66(8):905-14. doi: 10.1016/j.jacc.2015.06.1309. PMID: 26293760; PMCID: PMC5645077.
- Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, Hilfiker-Kleiner D, Sliwa K. Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart. 2013 Mar;99(5):308-13. doi: 10.1136/heartjnl-2012-302760. Epub 2012 Oct 31. PMID: 23118348.
- Nabbaale J, Okello E, Kibirige D, Ssekitoleko I, Isanga J, Karungi P, Sebatta E, Zhu ZW, Nakimuli A, Omagino J, Kayima J. Burden, predictors and short-term outcomes of peripartum cardiomyopathy in a black African cohort. PLoS One. 2020 Oct 21;15(10):e0240837. doi: 10.1371/journal.pone.0240837. PMID: 33085703; PMCID: PMC7577461.
- Ravi Kiran G, RajKumar C, Chandrasekhar P. Clinical and echocardiographic predictors of outcomes in patients with peripartum cardiomyopathy: A single centre, six month follow-up study. Indian Heart J. 2021 May-Jun;73(3):319-324. doi: 10.1016/j.ihj.2021.01.009. Epub 2021 Jan 6. PMID: 34154749; PMCID: PMC8322746.
- Nepal R, Bista M, Ghimire S. Early outcome of acute peripartum cardiomyopathy in eastern part of Nepal. Nepal Hear J. 2017; 14(1):13-15. doi:10.3126/njh.v14i1.17189
- Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, Wittstein IS, Dec GW, Zucker M, Narula J, Kip K, McNamara DM; IMAC2 Investigators. Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012 Jan;18(1):28-33. doi: 10.1016/j.cardfail.2011.09.009. Epub 2011 Nov 9. PMID: 22196838; PMCID: PMC3421073.
- Biteker M, Ilhan E, Biteker G, Duman D, Bozkurt B. Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur J Heart Fail. 2012 Aug;14(8):895-901. doi: 10.1093/eurjhf/hfs070. Epub 2012 May 15. PMID: 22588321.
- Hilfiker-Kleiner D, Meyer GP, Schieffer E, Goldmann B, Podewski E, Struman I, Fischer P, Drexler H. Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol. 2007 Dec 11;50(24):2354-5. doi: 10.1016/j.jacc.2007.10.006. Epub 2007 Nov 5. PMID: 18068047.
- Kearney L, Wright P, Fhadil S, Thomas M. Postpartum Cardiomyopathy and Considerations for Breastfeeding. Card Fail Rev. 2018; 4(2):112-118. doi:10.15420/cfr.2018.21.2
- Fett JD, Sannon H, Thélisma E, Sprunger T, Suresh V. Recovery from severe heart failure following peripartum cardiomyopathy. Int J Gynaecol Obstet. 2009 Feb;104(2):125-7. doi: 10.1016/j.ijgo.2008.09.017. Epub 2008 Nov 26. PMID: 19036370.
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz-Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Watkins H, Shah AJ, Seferovic PM, Elkayam U, Pankuweit S, Papp Z, Mouquet F, McMurray JJ; Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010 Aug;12(8):767-78. doi: 10.1093/eurjhf/hfq120. PMID: 20675664.
- Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, Czer LS, Illum S, Hatamizadeh P, Elkayam U. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009 Oct;15(8):645-50. doi: 10.1016/j.cardfail.2009.03.008. Epub 2009 Jul 16. PMID: 19786252.
- Goland S, Bitar F, Modi K, Safirstein J, Ro A, Mirocha J, Khatri N, Elkayam U. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail. 2011 May;17(5):426-30. doi: 10.1016/j.cardfail.2011.01.007. Epub 2011 Mar 11. PMID: 21549301.
- Demakis JG, Rahimtoola SH, Sutton GC, Meadows WR, Szanto PB, Tobin JR, Gunnar RM. Natural course of peripartum cardiomyopathy. Circulation. 1971 Dec;44(6):1053-61. doi: 10.1161/01.cir.44.6.1053. PMID: 4256828.
- O'Connell JB, Costanzo-Nordin MR, Subramanian R, Robinson JA, Wallis DE, Scanlon PJ, Gunnar RM. Peripartum cardiomyopathy: clinical, hemodynamic, histologic and prognostic characteristics. J Am Coll Cardiol. 1986 Jul;8(1):52-6. doi: 10.1016/s0735-1097(86)80091-2. PMID: 3711532.