More Information
Submitted: 23 October 2019 | Approved: 01 November 2019 | Published: 04 November 2019
How to cite this article: Kristinsdóttir H, Þórkelsson Þ, Harðardóttir H, Óskarsson G. Diagnosis of critical congenital heart defects in Iceland 2000-2014. J Cardiol Cardiovasc Med. 2019; 4: 177-181.
DOI: 10.29328/journal.jccm.1001062
ORCiD: orcid.org/0000-0002-7560949X
Copyright License: © 2019 Óskarsson G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Diagnosis of critical congenital heart defects in Iceland 2000-2014
Hallfríður Kristinsdóttir1, Þórður Þórkelsson1,2, Hildur Harðardóttir1,3 and Gylfi Óskarsson1,2*
1University of Iceland, Iceland
2Children’s Hospital, Landspitali University Hospital, Reykjavík, Iceland
3Department of Obstetrics and Gynecology, Landspitali University Hospital, Reykjavik, Iceland
*Address for Correspondence: Gylfi Óskarsson, Director of Pediatric Cardiology, Children’s Hospital, Landspitali University Hospital, University of Iceland, Reykjavík, Iceland, Tel: +354-8255019; Email: [email protected]
Critical congenital heart defects (CCHDs) are preferably diagnosed prenatally or soon after birth. Late diagnosis has been related to poorer prognosis. The aim of this study is to assess when CCHDs are diagnosed in Iceland and whether late diagnosis is a problem. All live born children in Iceland and foetuses diagnosed with CCHDs during the years 2000-2014 were included. CCHD was defined as a defect requiring intervention or causing death in the first year of life, or leading to abortion.
The total number of pre- and postnatal diagnosis of CCHDs was 188. Prenatal diagnosis was made in 69 of 188 (36.7%). Of 69 diagnosed prenatally 33 were terminated due to CCHD. Of the 155 live born children with CCHD, 36 (23.2%) had a prenatal diagnosis and 100 (64.5%) were diagnosed shortly after birth, before discharge from birth facility. 19 children (12.3%) were diagnosed late, that is after discharge from birth facility. Coarctation of the aorta was the most common CCHD diagnosed late (6/19).
Prenatal screening and newborn examination give good results in diagnosis of CCHDs in Iceland. Late diagnosis are relatively few, but both the number of prenatally diagnosed CCHDs and CCHDs diagnosed shortly after birth can be further improved.
The incidence of congenital heart defects in total is around 1%-1.7% [1-3]. About 10% of heart defects are critical, sometimes immediately life-threatening, and require early intervention (1-2.6 per 1000 live births) [4-8]. Early diagnosis of critical congenital heart defects (CCHDs), preferably prenatally or soon after birth, relates to a better outcome regarding survival and general prognosis [9-12].
Recent studies have shown that high proportion (13-30%) of CCHDs is diagnosed late, that is after discharge from birth facility [5-8,13,14]. The defects that are most often missed are left outflow tract obstructions (LOTOs), such as coarctation of the aorta (CoA) [7,8,14]. In response, many countries have implemented new methods to detect heart defects in newborn screening programmes. Routine pulse oximetry (POX) combined with physical examination is the most popular and most studied method, showing increased sensitivity and good specificity in diagnosis of CCHDs [4,15-18]. Still, POX also commonly misses LOTOs, for example CoA [4,15]. Newborn screening in Iceland includes a physical examination before discharge from birth facility, and a repeated physical examination at the age of 5 days. POX had not been introduced as a part of newborn screening during the study period. The aim of this study is to asses when CCHDs are diagnosed in Iceland and whether late diagnosis is a problem.
We conducted a retrospective study on all live births and abortions in Iceland from 2000 to 2014 (both years included) and included all diagnosed with CCHD. CCHD was defined as a structural defect of the heart or great vessels leading to abortion, requiring intervention, by open surgery or with catheterization, or causing death in the first year of life. Excluded were children delivered preterm (< 38 weeks gestation) with isolated patent ductus arteriosus.
Data on congenital heart disease diagnosis was collected from the National Hospital databases. Medical records of those with an ICD-10 diagnosis compatible with CCHD were reviewed to confirm a diagnosis of CCHD. Data on total live born children per year was obtained from the national birth registry and the general national registry. Data on follow-up was collected until and including January 2019. As this study was exclusively based on retrospective data collection no informed consent was needed and was not required by Landspitali National hospital bioethics committe. The study was performed according to national regulations and accepted by the Landspitali National hospital bioethics committe (3/2015).
Microsoft Office Excel 2007, R (version 3.1.2) and RStudio were used for statistical analysis, which was mostly descriptive. Chi-squared test was used for comparison of ratios and determined statistically significant if p < 0.05.
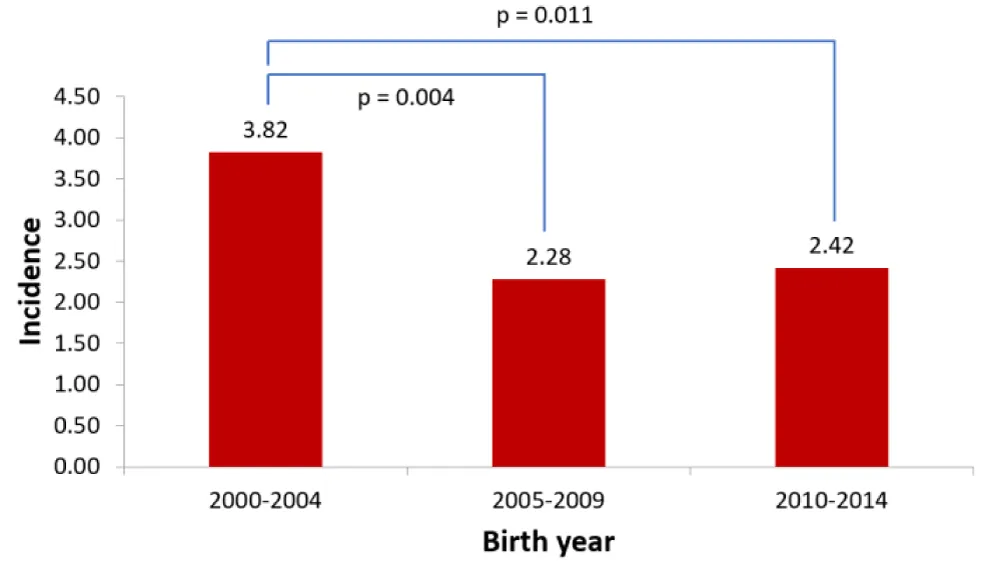
During the 15-year study period a total of 188 CCHDs were diagnosed pre- and postnatally. The incidence was 2.82 per 1000 live born children for the whole period. By dividing the study period in 5-year periods and comparing the incidence a significant reduction in incidence was found (Figure 1).
Figure 1: Comparison of incidence of CCHDs (including abortions) per 1000 live born in 5-year periods.
Prenatal diagnosis was made in 69 of 188 CCHDs or 36.7%. In 33 cases the family chose to terminate the pregnancy due to a poor prognosis of the diagnosed CCHD. A high proportion of HLHS cases, 19/21 were diagnosed prenatally, but no prenatal diagnosis of simple transposition of the great arteries (TGA) was achieved (0/15). The prenatal diagnosis are shown in table 1.
| Table 1: CCHDs diagnosed prenatally, in total, live born and aborted. | ||||||
| Total | Live born | Aborted | ||||
| Heart defect | Number (n = 69) |
% | Number (n = 36) |
% | Number (n = 33) |
% |
| HLHS | 19 | 27,5 | 1 | 2,8 | 18 | 54,5 |
| Complicated | 11 | 15,9 | 8 | 22,2 | 3 | 9,1 |
| CoA | 6 | 8,7 | 5 | 13,9 | 1 | 3,0 |
| CoA | 3 | 4,3 | 3 | 8,3 | 0 | 0,0 |
| CoA+VSD | 1 | 1,4 | 1 | 2,8 | 0 | 0,0 |
| CoA+subas | 1 | 1,4 | 1 | 2,8 | 0 | 0,0 |
| IAA+VSD | 1 | 1,4 | 0 | 0,0 | 1 | 3,0 |
| PA/IVS | 5 | 7,2 | 4 | 11,1 | 1 | 3,0 |
| AVSD | 4 | 5,8 | 1 | 2,8 | 3 | 9,1 |
| PA/VSD | 4 | 5,8 | 3 | 8,3 | 1 | 3,0 |
| SV | 3 | 4,3 | 0 | 0,0 | 3 | 9,1 |
| AS | 2 | 2,9 | 2 | 5,6 | 0 | 0,0 |
| DORV | 2 | 2,9 | 2 | 5,6 | 0 | 0,0 |
| Ebstein | 2 | 2,9 | 1 | 2,8 | 1 | 3,0 |
| PS | 2 | 2,9 | 2 | 5,6 | 0 | 0,0 |
| TGA(++) a | 2 | 2,9 | 2 | 5,6 | 0 | 0,0 |
| TOF | 2 | 2,9 | 2 | 5,6 | 0 | 0,0 |
| CCTGA+VSD+coa | 1 | 1,4 | 1 | 2,8 | 0 | 0,0 |
| TA | 1 | 1,4 | 0 | 0,0 | 1 | 3,0 |
| TA+PA | 1 | 1,4 | 0 | 0,0 | 1 | 3,0 |
| Truncus | 1 | 1,4 | 1 | 2,8 | 0 | 0,0 |
| VSD | 1 | 1,4 | 1 | 2,8 | 0 | 0,0 |
| a TGA(++) = TGA+VSD+1 other defect (CoA x1, and PS x1). | ||||||
The total number of live born children with CCHD was 155, an incidence of 2.33 per 1000. The male:female ratio was 93:62 or 1.5:1. A CCHD had been diagnosed prenatally in 36 of 155 live born children. Further 100 children (64.5%) were diagnosed before discharge home.
There were 19 children (12.3%) discharged home without a diagnosis of CCHD (Table 2). If CCHDs that are very difficult to detect early such as ALCAPA and double aortic arch are excluded, the number can be reduced to 16 children or 10,5%. 13 of 19 children had severe symptoms at diagnosis but late diagnosis did not cause any deaths. The most common CCHD to be diagnosed late was CoA, in 6 of the 19 children who were diagnosed after discharge home. Three of them were critically ill at diagnosis or in a state of circulatory shock. There was no significant difference in the number of late diagnosis between 5-year periods of the study.
| Table 2: CCHDs diagnosed late, after discharge home. | ||
| Heart defect | Number (n = 19) | % |
| CoA/IAA | 6 | 31.6 |
| CoA | 4 | 21.1 |
| CoA+VSD | 1 | 5.3 |
| IAA+VSD | 1 | 5.3 |
| ALCAPA | 2 | 10.5 |
| PDA | 2 | 10.5 |
| AVSD | 1 | 5.3 |
| DORV+VSD | 1 | 5.3 |
| MS | 1 | 5.3 |
| PbrS | 1 | 5.3 |
| SV | 1 | 5.3 |
| TGA(++) a | 1 | 5.3 |
| TOF | 1 | 5.3 |
| Double aortic arch | 1 | 5.3 |
| VSD | 1 | 5.3 |
| a TGA(++) = TGA+VSD+DORV. | ||
The most common CCHDs in the total cohort were CoA (32/188), VSD (24/188) and HLHS (21/188). An overview of all diagnosis is shown in table 3.
| Table 3: All diagnosis of CCHDs. | ||
| Heart defect | Number (n = 188) | % |
| CoA | 32 | 17,0 |
| CoA | 19 | 10,1 |
| CoA+VSD | 8 | 4,3 |
| IAA+VSD | 2 | 1,1 |
| CoA+as | 1 | 0,5 |
| CoA+subas | 1 | 0,5 |
| CoA+subas+VSD | 1 | 0,5 |
| VSD | 24 | 12,8 |
| HLHS | 21 | 11,2 |
| TGA | 21 | 11,2 |
| TGA | 12 | 6,4 |
| TGA+VSD | 3 | 1,6 |
| TGA(++) a | 6 | 3,2 |
| TOF | 13 | 6,9 |
| Complicated | 12 | 6,4 |
| AVSD | 9 | 4,8 |
| PS | 8 | 4,3 |
| AS | 8 | 4,3 |
| AS | 6 | 3,2 |
| AS+coa | 2 | 1,1 |
| PA/IVS | 6 | 3,2 |
| PA/VSD | 6 | 3,2 |
| Truncus | 5 | 2,7 |
| Truncus | 4 | 2,1 |
| Truncus+IAA | 1 | 0,5 |
| SV | 4 | 2,1 |
| DORV | 4 | 2,1 |
| ALCAPA | 2 | 1,1 |
| Ebstein | 2 | 1,1 |
| PDA | 2 | 1,1 |
| APW | 1 | 0,5 |
| CCTGA+VSD+coa | 1 | 0,5 |
| Coronary fistula | 1 | 0,5 |
| Ductal aneurysm | 1 | 0,5 |
| MS | 1 | 0,5 |
| PbrS | 1 | 0,5 |
| TA | 1 | 0,5 |
| TA+PA | 1 | 0,5 |
| Double aortic arch | 1 | 0,5 |
| a TGA+VSD+1 other defect (CoA x2, PS or PS+PIS x2, MS x1, and DORV x1). | ||
Of the 155 live born children 20 had genetic diagnosis. The most common were trisomy 21 (9), XO (2) and microdeletion of chromsome 22 (2). Among the 36 terminated pregnancies two fetuses had trisomy 21, two trisomy 13, and further two other chromosomal abnormalities.
Of 155 live born children with CCHD 5 children could not be offered operation or intervention either due to the severity of the CCHD, serious chromosomal defects, prematurity or neurological problems and all 5 died. Of the remaining 150 children, 117 had surgery performed, and 33 interventional heart catheterisation, all before the age of 1 year. The surgeries and interventional heart catheterisations were performed in Iceland, Boston (USA), Lund (Sweden), or London (UK).
At the end of the follow-up period 141 of 150 children who were eligible for surgical or interventional treatment are alive (94%), at the age of 4-19 years. Nine children died after surgical or interventional treatment for CCHD was performed. Of those two children (1.3%) died within 30 days after the procedure. One died after interventional ballon dilatation of valvular pulmonary stenosis, and one after surgical atrial septectomy and Blalock Taussig shunt procedure for pulmonary atresia with intact ventricular septum. Three children died of multiple problems caused by genetic disorders. Two children died of late complications of complex CCHD. Further two children died of other or unknown causes not related to their CCHD.
This nationwide study covers a 15-year period and illustrates the incidence, timing of diagnosis and results of treatment of CCHDs. The incidence of 2.33/1,000 live born is similar to the incidence of 1.9-2.6/1000 found in previous studies that also include CCHDs requiring intervention in the first year of life [4-6]. The observed decrease in incidence during the study period might be related to the implementation of a nationwide but optional first trimester screening for chromosomal abnormalities in the year 2003. This program has led to increased early detection and abortion of foetuses with serious chromosomal defects such as Down syndrome where CCHDs are common [19,20].
Prenatal diagnosis was made in 37% of the total cohort. Almost half of those diagnosed prenatally had so serious CCHD that abortion was chosen. The remaining CCHDs diagnosed prenatally composed 23% of live born children with CCHD. The proportion of prenatal diagnosis is similar to many previous studies, but recent studies are increasingly showing higher proportions of prenatal diagnosis, or 50%-75% [17,21,22]. Our results in prenatal diagnosis of very severe CCHDs such as HLHS with poor prognosis are good. There is however room for improvement in prenatal diagnosis of CCHDs that are serious but seldom lead to abortion, such as TGA, where no prenatal detection was achieved during the study period. During the study period systematic examination of the outflow tracts and three vessel view was not performed at screening foetal ultrasound. Implementation of these techniques would be necessary in order to improve our results of prenatal screening for CCHDs [23].
As the majority of children with CCHD are not diagnosed prenatally it is important to organize effective methods to detect children with CCHD early, before they show serious symptoms. During the study period newborn children were examined twice at the age of 1 and 5 days. POX screening was not performed. By these means only 12.3% of live born children with CCHD were diagnosed late, after discharge home.
Some of the late diagnosed CCHDs (shown in table 2) are very difficult to detect early, such as ALCAPA and double aortic arch. Late diagnosis of other CCHDs could probably be reduced with methods such as POX. At least 3 children with cyanotic heart disease in our material probably would have been diagnosed earlier if POX had been applied. If these diagnosis had been detected early, the proportion of late diagnosis would have been reduced to 10,3%, which compares well with previous studies showing very few late diagnosis [16].
The most common late diagnosis in our study was CoA, where late diagnosis is a universal problem with or without POX screening [4,7,8,14,15]. The number of CoA in the total cohort was 32 and 6 (18.8%) of those were diagnosed late. While more than 80% of CoA in our study were diagnosed prenatally or shortly after birth the fact that half of the children (3 of 6) with late diagnosis of CoA were critically ill and in a state of circulatory shock highlights the importance of trying to identify these children early.
The impact of CCHDs on survival is clear from our study. Of 188 pre- and postnatal diagnosis of CCHD 47 have led to termination of pregnancy or death (25%). CCHDs with poor prognosis such as HLHS where two chamber correction is not possible account for the majority of those. The survival of those that were offered surgery or catheter intervention is however acceptable. In-hospital mortality was 1.3% which compares favourably with previous studies [24,25]. Later mortality after surgery or intervention occurred in 7 of 148 children, but the majority of those could be explained by serious comorbidities, and only two directly to the CCHD.
The organisation of pre- and postnatal diagnosis of CCHDs in Iceland during the period of 2000-2014 resulted in a high proportion of children with very serious CCHD and poor prognosis to be detected prenatally. The children with CCHD not diagnosed prenatally were most commonly diagnosed early after birth, and late diagnosis after discharge home from birth facility were relatively few. Late diagnosis of CCHD did not result in any deaths. The results of pre- and postnatal detection of CCHDs can be improved by refining the methods for prenatal foetal echocardiography and by adding POX screening of newborns. Both changes have been implemented, and the decisions were supported by the data presented.
- van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, et al. Birth Prevalence of Congenital Heart Disease Worldwide: A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2011; 58: 2241-2247. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22078432
- Leirgul E, Fomina T, Brodwall K, Greve G, Holmstrøm H, et al. Birth prevalence of congenital heart defects in Norway 1994-2009-A nationwide study. Am Heart J. 2014; 168: 956-964. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25458661
- Stephensen SS, et al. Nýgengi og greining meðfæddra hjartagalla á Íslandi 1990-1999. Læknablaðið. 2002; 88: 281-287.
- Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011; 378: 785-794. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21820732
- Dawson AL, Cassell CH, Riehle-Colarusso T, Grosse SD, Tanner JP, et al. Factors Associated With Late Detection of Critical Congenital Heart Disease in Newborns. Pediatrics. 2013; 132: E604-E611. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23940249
- Liberman RF, Getz KD, Lin AE, Higgins CA, Sekhavat S, et al. Delayed Diagnosis of Critical Congenital Heart Defects: Trends and Associated Factors. Pediatrics. 2014; 134: E373-E381. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25070301
- Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008; 93: F33-F35. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17556383
- Mouledoux JH, Walsh WF. Evaluating the Diagnostic Gap: Statewide Incidence of Undiagnosed Critical Congenital Heart Disease Before Newborn Screening With Pulse Oximetry. Pediatr Cardiol. 2013; 34: 1680-1686. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23595939
- Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, et al. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006; 92: 1298-1302. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16449514
- Bonnet D, Coltri A, Butera G, Fermont L, Le Bidois J, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999; 99: 916-918. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10027815
- Calderon J, Angeard N, Moutier S, Plumet MH, Jambaqué I, et al. Impact of Prenatal Diagnosis on Neurocognitive Outcomes in Children with Transposition of the Great Arteries. J Pediatr. 2012; 161: 94-98. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22284567
- van Velzen CL, Haak MC, Reijnders G, Rijlaarsdam ME, Bax CJ, et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Obstet Gynecol. 2015; 45: 320-325. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25297053
- Mellander M, Sunnegardh J. Failure to diagnose critical heart malformations in newborns before discharge--an increasing problem? Acta Paediatr. 2006; 95: 407-413. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16720486
- Peterson C, Ailes E, Riehle-Colarusso T, Oster ME, Olney RS, et al. Late Detection of Critical Congenital Heart Disease among US Infants Estimation of the Potential Impact of Proposed Universal Screening Using Pulse Oximetry. Jama Pediatrics. 2014; 168: 361-370. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24493342
- de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; 338: 3037. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19131383
- Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012; 379: 2459-2464. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22554860
- Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, et al. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine-results from a prospective multicenter study. Eur J Pediatr. 2010; 169: 975-981. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20195633
- Meberg A, Andreassen A, Brunvand L, Markestad T, Moster D, et al. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr. 2009; 98: 682-686. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19154526
- Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998; 80: 213-217. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9843040
- Irving CA, Chaudhari MP. Cardiovascular abnormalities in Down's syndrome: spectrum, management and survival over 22 years. Arch Dis Child. 2012; 97: 326-330. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21835834
- Carvalho JS, Allan LD, Chaoui R, Copel JA, DeVore GR, et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013; 41: 348-359. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23460196
- Chew C, Halliday JL, Riley MM, Penny DJ. Population-based study of antenatal detection of congenital heart disease by ultrasound examination. Ultrasound Obstet Gynecol. 2007; 29: 619-624. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17523161
- Carvalho JS, Mavrides E, Shinebourne EA, Campbell S, Thilaganathan B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart. 2002; 88: 387-391. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12231598
- Hoashi T, Miyata H, Murakami A, Hirata Y, Hirose K, et al. The current trends of mortality following congenital heart surgery: the Japan Congenital Cardiovascular Surgery Database. Interact Cardiovasc Thorac Surg. 2015; 21: 151-156. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25924867
- Rogers L, Brown KL, Franklin RC, Ambler G, Anderson D, et al. Improving Risk Adjustment for Mortality After Pediatric Cardiac Surgery: The UK PRAiS2 Model. Ann Thorac Surg. 2017; 104: 211-219. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28318513