More Information
Submitted: January 10, 2021 | Approved: November 30, 2021 | Published: December 01, 2021
How to cite this article: Cowgill JA, Moran AM. Stiff “Left Atrial” syndrome post-mustard procedure. J Cardiol Cardiovasc Med. 2021; 6: 069-073.
DOI: 10.29328/journal.jccm.1001122
Copyright License: © 2021 Cowgill JA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Stiff “Left Atrial” syndrome post-mustard procedure
Joshua A Cowgill1 and Adrian M Moran2*
1Department of Cardiology and Cardiovascular Medicine, Maine Medical Center, 22 Bramhall St, Portland Maine, 04102, USA
2Department of Pediatrics and Cardiology, Barbara Bush Children’s Hospital, Maine Medical Center, 22 Bramhall St, Portland Maine, 04102, USA
*Address for Correspondence: Adrian M Moran, M.B.B.Ch, B.A.O., M.B.A., F.A.C.C. Department of Pediatrics and Cardiology, Barbara Bush Children’s Hospital, Maine Medical Center, 22 Bramhall St, Portland Maine, 04102, USA, Email: [email protected]
Objectives: We describe the clinical course and management of two patients with post-capillary pulmonary hypertension due to diffuse pulmonary venous baffle calcification decades post-Mustard procedure.
Background: From the late 1950s to the early 1990s, the definitive surgical repair for children with D-transposition of the great vessels (D-TGA) was an atrial switch procedure (either Senning or Mustard operation) which utilizes atrial-level baffles to shunt pulmonary venous blood to the morphologic right (systemic) ventricle and caval blood to the morphologic left (sub-pulmonary) ventricle. From a hemodynamic standpoint, baffle leaks and stenoses as well as precapillary pulmonary hypertension have all been described as both early and late complications [1]. Recently, delayed post-capillary pulmonary hypertension (in the absence of discrete baffle obstruction) decades post-atrial switch has also been described [2]. The underlying pathophysiology for this postcapillary pulmonary hypertension is unclear but is theorized to involve impaired diastology referable to the pulmonary venous baffle.
Methods/Results: Using hemodynamic and imaging data, we describe two patients with extensive pulmonary venous baffle calcification and resultant pulmonary hypertension from the so-called “stiff left atrial (LA) syndrome.” This problem can be difficult to treat medically and is not amenable to catheter-based interventions. We hypothesize that this is an underlying mechanism for pulmonary hypertension in at least some post-Mustard and Senning patients.
Conclusion: We describe the treatments and clinical course for each of these patients, and in particular describe how the surgical revision of the pulmonary venous baffle in one case led to the complete resolution of symptoms.
Until the advent of the arterial switch operation in the late 1980s, the definitive surgical repair for patients with D-transposition of the great vessels (D-TGA) was an atrial switch procedure (either Senning or Mustard procedures). Atrial switches utilize atrial-level prosthetic or pericardial baffles to shunt pulmonary venous blood across the tricuspid valve to the right (systemic) ventricle and, vice versa, to shunt systemic venous return across the mitral valve to the left (sub-pulmonary) ventricle. Baffle leaks and discrete obstructions are well described short and long term complications of atrial switch procedures1, and they are often amenable to percutaneous catheter directed interventions. We will describe two patients with recalcitrant pulmonary hypertension due to diffuse and extensive calcification of the pulmonary venous baffle (neo-left atrium) decades post-Mustard. This phenomenon is not described in the literature, is not particularly amenable to catheter-based interventions, and poses significant difficulty in symptom management.
Patient 1
Patient 1 is a 44-year-old man with a history of D-TGA palliated who underwent a Blalock-Hanlon atrial septectomy as a neonate and Mustard procedure (atrial switch procedure with dacron-constructed baffles) at the age of 2 years. His clinical course throughout adulthood included a combined restrictive and obstructive pulmonary disease, intermittent intra-atrial reentrant tachycardia, and sinus node dysfunction eventually resulting in pacemaker implantation at the age of 31 years. He had been diagnosed with pulmonary artery hypertension (WHO group I PAH, secondary to congenital heart disease) at the age of 12 years, but this was clinically well tolerated for most of his life without the need for specific pulmonary vasodilators or chronic diuretics. He was lost to follow up for a number of years. At the age of 43 he developed progressive dyspnea on exertion and lower extremity edema. Echocardiogram demonstrated Mustard anatomy, mild to moderate right ventricular and left ventricular dysfunction, trivial mitral and tricuspid regurgitation, severely dilated main pulmonary artery (4.5 cm), and suspicion for pulmonary venous baffle obstruction near the baffle crux.
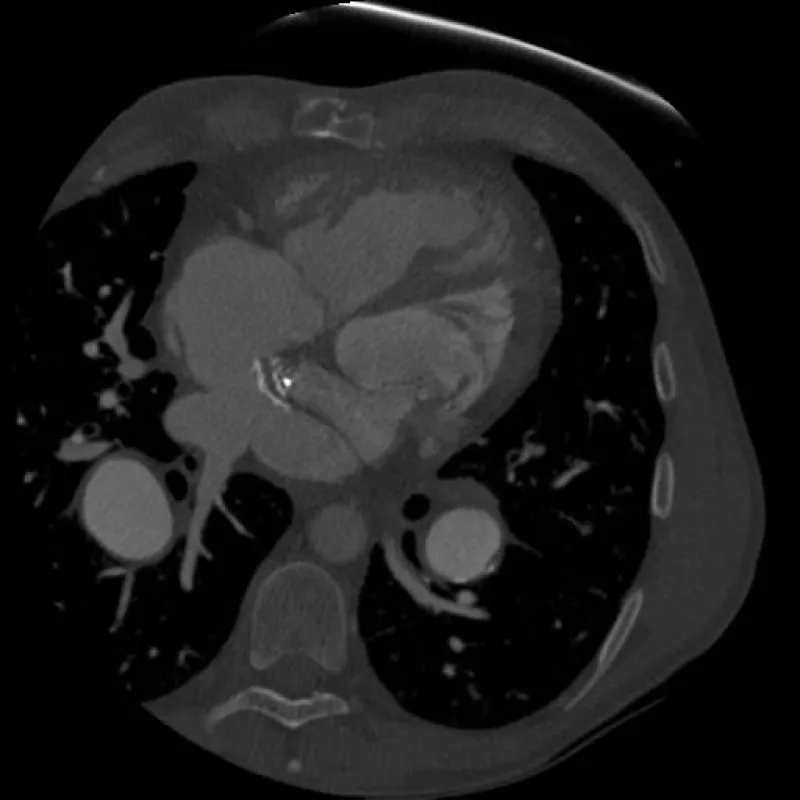
Cardiac catheterization (Table 1) demonstrated severe pulmonary hypertension with mean pulmonary artery pressure of 64 mmHg and a 16 mmHg gradient between pulmonary capillary wedge pressure (PCWP) and his RV (systemic ventricle) end diastolic pressure. He was not vasodilator responsive. Echocardiography and initial catheterization findings raised concern for baffle obstruction causing severe fixed pulmonary hypertension as a means to explain the documented gradient. In addition to his known underlying elevated pulmonary vascular resistance (PVR). He underwent re-intervention using a Brockenbrough technique to directly access the pulmonary venous baffle, confirm a baffle gradient with the intention of stenting the obstructed baffle. During this second catheterization, direct pressure measurements of the pulmonary venous baffle revealed no discrete baffle stenosis. It showed that there was no pressure gradient from the pulmonary veins to the tricuspid valve. Rather, direct pressures measurements revealed that direct A wave pressure in the baffle was 23 mmHg and commensurate with the RV end diastolic pressure (RVEDP), while the V wave was greatly elevated to 35 mmHg (tricuspid regurgitation was trivial by transesophageal echocardiographic assessment). Given the echocardiographic appearance of mild narrowing, the area in question was balloon-dilated without waist formation seen on angiography. Instead the neo left atrium was deemed rigid and undersized. Taken together, pulmonary hypertension with a large PCWP to RVEDP gradient, in the absence of a discrete hemodynamically significant obstruction, and with very large V waves in direct pressure measurement of the pulmonary venous baffle raised the concern for a diffusely stiff and calcified chamber with very poor compliance as the primary cause of his severe pulmonary hypertension. To confirm this diagnosis, the patient underwent a cardiac gated CT angiogram of the chest (Figure 1). CT showed the pulmonary venous baffle was patent but heavily calcified. Using cine imaging, the entire atrial/baffle complex was noted to be essentially non-compliant and moved en bloc with the beating ventricles.
| Table 1: Invasive hemodynamic data for patient 1. | |
| PA pressure | 86/48 (mean 64) mmHg |
| PCWP | 31/39 (35) mmHg |
| RVEDP | 19 mmHg |
| Pulmonary vascular resistance (PVR) | 11.8 Woods units |
| Transpulmonary gradient (mean PA to PCWP) | 29 mmHg |
| PCWP A wave to RVEDP gradient | 12 mmHg |
| Pulmonary venous baffle pressure (subsequent procedure) | 21/35 (21) mmHg |
Figure 1: CT image demonstrating heavily calcified pulmonary venous baffle. Calcification is heaviest on the baffle’s anterior aspect at the level of the systemic and pulmonary venous baffle crux.
Given this “fixed” left atrial hypertension, it was felt that pulmonary vasodilators to alleviate his elevated PVR would be potentially harmful and could create significant pulmonary edema. Instead the patient was treated with cautious diuresis and afterload reduction with an angiotensin-receptor blocker. Over a period of several months he improved symptomatically and was able to resume his work and exercise; however, he did not fully return to his previous functional level. The potential for a reconstructive surgery of his atrial/baffle complex was discussed both with the patient and congenital heart surgeons. Ultimately given his severe elevation in PVR he was deemed not to be a candidate for open baffle revision, with heart lung transplantation his only surgical option (which is being considered).
Patient 2
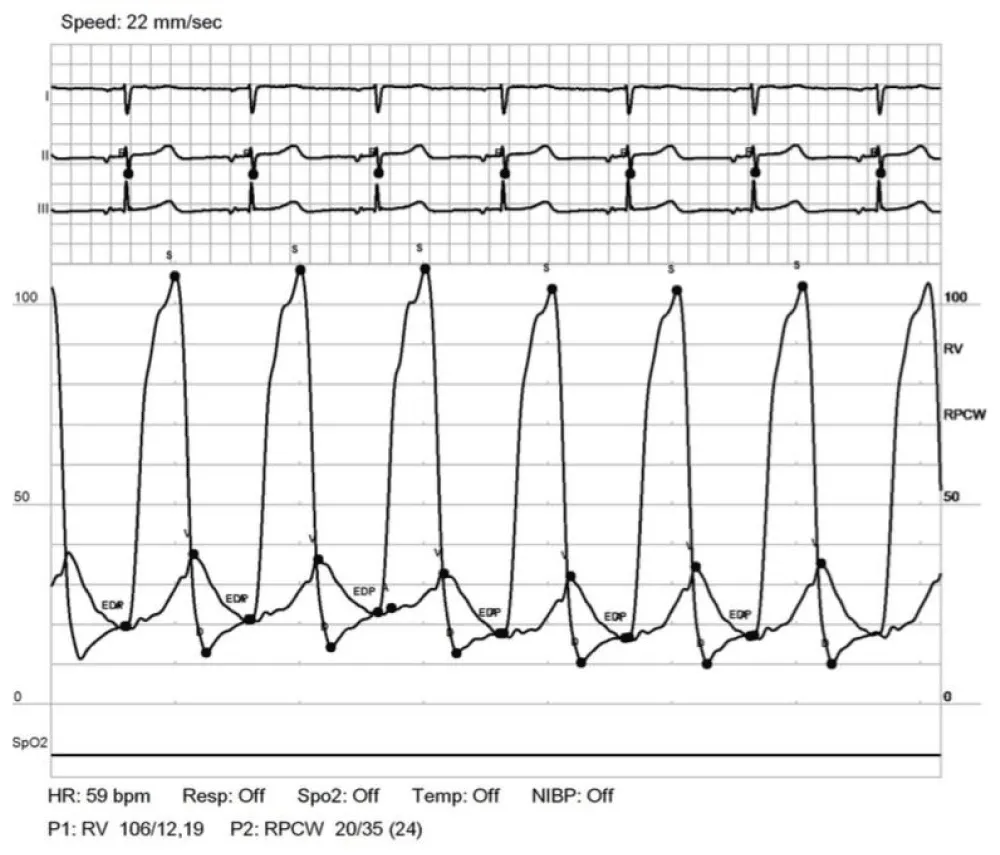
Patient 2 is a 34-year-old woman with a history of D-TGA palliated with a Rashkind atrial septostomy as a neonate and eventually a Mustard procedure (atrial-switch operation with dacron-constructed baffles) at the age of 11 months. Due to obstruction of the systemic venous baffle, she underwent a baffle reconstruction with Gore-tex material at the age of 7 years. She was monitored for long-standing (> 20 years) mild pulmonary venous baffle obstruction (mean 4-5 mmHg gradient across the baffle by serial echo) without significant pulmonary hypertension. Serial echocardiograms noted that the pulmonary venous baffle was diffusely “echo bright” and was presumed secondary to calcification. The gradient across the pulmonary venous baffle did worsen (to mean 9-15 mmHg) during her two successful pregnancies, requiring peripartum diuresis. After each delivery gradients returned to baseline and diuretics were discontinued. At the age of 32 years (4 years postpartum), she developed slowly progressive, severe exercise intolerance and a feeling of chest heaviness. Echocardiogram at that time demonstrated Mustard anatomy, low/normal RV function, mild tricuspid regurgitation, and the known diffusely calcified pulmonary venous baffle with mean pressure gradient of 4-6 mmHg and peak of 22 mmHg through the baffle. Cardiac catheterization (Table 2) demonstrated mild pulmonary hypertension with mean pulmonary artery pressure of 32 mmHg and, as with patient 1, markedly elevated V waves on pulmonary artery wedge tracings. PCWP A waves were measured at 20 mmHg and commensurate with the RVEDP of 19 mmHg; V waves were 35 mmHg (Figure 2).
| Table 2: Invasive hemodynamic data for patient 2. | |
| PA pressure | 48/18 (mean 32) mmHg |
| PCWP | 20/35 (24) mmHg |
| RVEDP | 19 mmHg |
| Pulmonary vascular resistance (PVR) | 4 Woods units |
| Transpulmonary gradient (mean PA to PCWP) | 8 mmHg |
| PCWP A wave to RVEDP gradient | 0 mmHg |
Figure 2: Simultaneous RV and PCWP tracing for patient 2. Tracings demonstrate A wave pressure that correlates with RVEDP and a very large V wave.
Without discrete obstruction (PCWP A wave = RVEDP), intervention on the pulmonary venous baffle was not pursued. Like patient 1, this patient was treated symptomatically with cautious diuresis and afterload reduction with ACE inhibitor. Over the next two years she slowly developed progressive exercise intolerance and a feeling of chest heaviness. Follow up echocardiography showed a worsened pulmonary venous baffle gradient to mean of 11 mmHg and peak of 30 mmHg, with continued mild tricuspid regurgitation (TR) and low/normal RV function. Based on her symptomatology and echo evidence of worsening baffle obstruction, she was referred to an outside institution for potential baffle intervention. Cardiac catheterization demonstrated similar pressure gradients from 2 years prior but also showed calcification of the pulmonary venous baffle with a mild discrete narrowing just proximal to the tricuspid valve, as well as a pressure gradient of 6 mmHg across the tricuspid valve (which in retrospect was due to a prominent V wave). Given her symptomatology, balloon dilation of both the stenotic baffle and the tricuspid valve itself was attempted. Unfortunately, the procedure was complicated by balloon rupture without distal embolization due to the heavily calcified baffle. Femoral artery cutdown was needed to remove the device. The patient underwent re-intervention 7 days later with successful technical completion of the balloon dilation of the stenotic portion of the pulmonary venous baffle and device closure of the Brockenbrough-induced atrial septal defect.
Her post-procedural course was complicated by a wound infection at the site of the femoral cutdown, but she otherwise demonstrated some modest improvement from a cardiopulmonary standpoint in the immediate post-procedural period. Follow-up echo 2 weeks post-procedure showed continued turbulent flow across the pulmonary venous baffle, continued low/normal RV function, and new evidence of moderate TR. Over the next 4 months she developed debilitating dyspnea on exertion, fatigue, and lightheadedness. Serial echocardiography showed progression of her TR into the severe range. Bacterial endocarditis was ruled out and the patient was re-evaluated for intervention on the tricuspid valve. After discussion with the patient of the profound risk of the operation, she underwent surgical placement of a mechanical valve in the tricuspid position. Additionally, the pulmonary venous baffle was confirmed to have heavy calcification throughout the structure and was undersized limiting pulmonary venous return; it was therefore augmented with new Gore-tex baffle. Her post-operative course was uncomplicated. Over the subsequent months, her shortness of breath and overall fatigue improved dramatically, she was weaned off diuretics and afterload reduction, and she was able to resume her work. Follow-up echocardiogram demonstrates continued low/normal RV function, well-functioning prosthetic tricuspid valve, and minimal flow gradient across the new pulmonary venous baffle and tricuspid valve (peak gradient 11 mmHg and mean gradient 3 mmHg) (Table 3). Follow up catheterization has been declined by the patient.
| Table 3: Timeline of baffle gradients for patient 2. | |
| Serial childhood transthoracic echocardiogram (TTE) | Mean 4-5 mmHg |
| Onset of symptoms TTE | Mean 6; peak 22 mmHg |
| Onset of symptoms catheterization | 0 mmHg |
| 2 year follow up TTE | Mean 11; peak 30 mmHg |
| Post-baffle balloon dilation TTE | Unable to assess in the setting of severe TR |
| Post-tricuspid valve replacement and baffle reconstruction TTE | Mean 3; peak 11 mmHg |
Baffle obstruction due to discrete stenosis and baffle leaks are well described short and long term complications of the Mustard procedure1, but, to our knowledge, extensive calcification of the entire chamber and resultant non-compliance of the neo-left atrium decades post-Mustard has not been described in the literature. A discrete stenosis and obstruction of the baffle is often amenable to catheter directed intervention via balloon dilation or stent (as was pursued initially in both of our patients). The involvement of the entire chamber, however, requires an open baffle revision if symptoms are not controllable with medical therapy.
While delayed precapillary pulmonary hypertension is fairly well described in this patient population, and our patient 1 had some degree of group I PAH, recently Chaix and colleagues have described a phenomenon of delayed postcapillary pulmonary hypertension in a large cohort of adults many years post-atrial switch for D-TGA who specifically did not have discrete baffle stenosis [2]. The underlying pathophysiology is only hypothesized at this point, but the authors opine that it is likely “impaired diastology resulting from a rigid atrial baffle plays a role [2]”. The diffuse baffle calcification we describe is potentially an underlying mechanism for at least some of these patients. A similar phenomenon to what we describe is the so-called “stiff LA syndrome” that has been described in adults after mitral valve surgery [3], extensive catheter ablation for atrial fibrillation [4], or post-Maze procedure for the same [5]. Stiff LA syndrome is characterized by dyspnea, non-arteriolar pulmonary hypertension, and very large V waves on pulmonary wedge or direct LA pressure measurement in the absence of significant mitral regurgitation [6]. While the syndrome is well described at this point, the treatment is not. Surgical “revision” of a native LA is not a feasible approach in the case of post-ablation or post-Maze stiff LA (with diuresis being the mainstay of treatment), but baffle revision may be a consideration post-Mustard as we have described. Open baffle revision is a high-risk procedure in the post-Mustard population who often have some degree of RV (systemic ventricle) dysfunction and are predisposed to multiple arrhythmias, but it can be considered in those with severe symptoms recalcitrant to medical therapy.
With regards to medical therapy, studies of stiff LA syndrome have shown benefit with diuresis [5,6] and similar clinical responses were noted in our patients. The use of the oral pulmonary vasodilator sildenafil has also been described in stiff LA syndrome post-atrial fibrillation ablation [7]. However, we have concerns that in the post-Mustard population with fixed pulmonary hypertension these drugs could precipitate significant pulmonary edema – similar to the phenomenon seen in pulmonary veno-occlussive disease where pulmonary vasodilators are considered contraindicated [8-10].
We describe two patients with diffuse and heavy pulmonary venous baffle calcification resulting in a stiff, non-compliant neo-left atrium and pulmonary hypertension greater than 30 years post-Mustard procedure for D-TGA. In patient 1, we describe the phenomenon from a hemodynamic standpoint (mixed arteriolar and non-arteriolar pulmonary hypertension with very large baffle V waves and no discrete obstruction across the baffle), as well as cardiac-gated CT confirmation (heavily calcified, non-compliant baffle that moves en bloc with the beating ventricle). In patient 2, we re-demonstrate the hemodynamic characteristics of this phenomenon, and though her surgery was ultimately undertaken due to a complication of cardiac catheterization, the revision of her pulmonary venous baffle with neo-left atrial enlargement and subsequent complete resolution of symptoms, serves as proof-of-concept that this degree of chamber non-compliance is causative of severe symptomatology and surgery can be curative. We believe that without baffle enlargement that patient 2 would have suffered continued symptoms similar to those prior to their catheterization.
This phenomenon is one that any provider caring for adult patients with congenital heart disease should be familiar with. Non-arteriolar pulmonary hypertension with very large V waves seen in cardiac catheterization is the most important clue to diagnosis. Initial medical therapy is supportive, but we would advise extreme caution with pulmonary vasodilators as they may precipitate significant pulmonary edema in these patients. Surgical revision of the pulmonary venous baffle, while high-risk, can be curative and should be considered after appropriate medical therapy fails.
- Vejlstrup N, Sørensen K, Mattsson E, Thilén U, Kvidal P, et al. Long-Term Outcome of Mustard/Senning Correction for Transposition of the Great Arteries in Sweden and Denmark. Circulation. 2015; 132: 633-638. PubMed: https://pubmed.ncbi.nlm.nih.gov/26185211/
- Chaix MA, Dore A, Mercier LA, Mongeon FP, Marcotte F, et al. Late Onset Postcapillary Pulmonary Hypertension in Patients With Transposition of the Great Arteries and Mustard or Senning Baffles. J Am Heart Assoc. 2017; 6: e006481. PubMed: https://pubmed.ncbi.nlm.nih.gov/29025749/
- Pilote L, Hüttner I, Marpole D, Sniderman A. Stiff left atrial syndrome. Can J Cardiol. 1988; 4: 255-257. PubMed: https://pubmed.ncbi.nlm.nih.gov/3179789/
- Yang Y, Liu Q, Wu Z, Li X, Xiao Y, et al. Stiff Left Atrial Syndrome: A Complication Undergoing Radiofrequency Catheter Ablation for Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016; 27: 884-889. PubMed: https://pubmed.ncbi.nlm.nih.gov/26920815/
- Welch TD, Coylewright M, Powell BD, Asirvatham SJ, Gersh BJ, et al. Symptomatic pulmonary hypertension with giant left atrial v waves after surgical maze procedures: evaluation by comprehensive hemodynamic catheterization. Heart Rhythm. 2013; 10: 1839-1842. PubMed: https://pubmed.ncbi.nlm.nih.gov/24050987/
- Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011; 8: 1364-1371. PubMed: https://pubmed.ncbi.nlm.nih.gov/21354332/
- Wong GR, Lau DH, Baillie TJ, Middeldorp ME, Steele PM, et al. Novel use of sildenafil in the management of pulmonary hypertension due to post-catheter ablation “stiff left atrial syndrome”. Int J Cardiol. 2015; 181: 55-56. PubMed: https://pubmed.ncbi.nlm.nih.gov/25482279/
- O’Callaghan DS, Dorfmuller P, Jaïs X, Mouthon L, Sitbon O, et al. Pulmonary veno-occlusive disease: the bête noire of pulmonary hypertension in connective tissue diseases? Presse Medicale Paris Fr 1983. 2011; 40: e65-78. PubMed: https://pubmed.ncbi.nlm.nih.gov/21211937/
- Masters K, Bennett S. Pulmonary veno-occlusive disease: an uncommon cause of pulmonary hypertension. BMJ Case Rep. 2013; 2013: bcr2012007752. PubMed: https://pubmed.ncbi.nlm.nih.gov/23378546/
- Hansmann G. Pulmonary Hypertension in Infants, Children, and Young Adults. J Am Coll Cardiol. 2017; 69: 2551-2569. PubMed: https://pubmed.ncbi.nlm.nih.gov/28521893/