More Information
Submitted: November 24, 2022 | Approved: December 01, 2022 | Published: December 02, 2022
How to cite this article: Zaoui N, Boukabous A, Bachir N, Terki A, Irid N. Percutaneous Atrial Septal Defect (ASD) closure technique in case of association with an azygos continuation of the inferior vena cava “case report”. J Cardiol Cardiovasc Med. 2022; 7: 104-108.
DOI: 10.29328/journal.jccm.1001143
Copyright License: © 2022 Zaoui N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Case report; ASD; Congenital heart disease; Percutaneous closure; Internal jugular vein
Percutaneous Atrial Septal Defect (ASD) closure technique in case of association with an azygos continuation of the inferior vena cava “case report”
Nassime Zaoui*, Amina Boukabous, Nadhir Bachir, Ali Terki and Nabil Irid
Cardiology Department, Omar Yacef Draa Ben Khedda Hospital, Tizi-Ouzou Medical University, Algeria
*Address for Correspondence: Nassime Zaoui, Cardiology Department, Omar Yacef Draa Ben Khedda Hospital, Tizi-Ouzou Medical University, Algeria, Email: [email protected]
Introduction: Atrial Septal Defect (ASD) is the most common congenital heart disease, accessible to percutaneous closure in 90% of cases. The closure procedure is performed usually under local anesthesia and TTE by femoral access. The association of OS-ASD with an azygos continuation of the inferior vena cava is very rare (< 0.1/1000 births) making femoral access impossible. Only a few cases are mentioned in the literature, here we describe the procedure as faithfully as possible.
Important clinical finding: We present a case of a 32-years-old female candidate for percutaneous closure of OS-ASD with right cavity dilatation who present during her procedure an unusual guidewire path suspecting an azygos continuation of the inferior vena cava, confirmed by CT angiography, making impossible the closure via the femoral approach.
Therapeutic intervention: After being confronted with the categorical patient refusal of the surgery, we performed successfully the procedure; one month later; under general sedation by internal jugular approach. We finished with manual compression before extubating the patient.
Outcomes: The follow-up was favorable at the cost of a hematoma at the puncture site and brachial plexus compression, which regressed after 3 days.
Conclusion: We opted for general anesthesia and intubation to guide the procedure by TEE. We placed it in the aorta, which gave us good stability to continue successfully the procedure. We underestimated the risk of complication at the puncture site, which could have been avoided by using a vascular suture device or more prolonged compression.
Main takeaway lesson: Percutaneous closure is the reference treatment for OS-ASD. In case of is associated with an azygos continuation of the inferior vena cava, the right internal jugular vein remains a reasonable approach; it requires discussion and rigorous preparation by the whole team. The management of the puncture site in this situation remains delicate and requires great concentration.
ASD: Atrial Septal Defect; LA: Left Atrium; LV: Left Ventricle; M: Month; MPA: Multipurpose A; OS: Ostium Secundum; RV: Right Ventricle; TEE: Transesophageal Echocardiography; TTE: Transthoracic Echocardiography
Atrial septal defect (ASD) is the most common congenital heart disease; its prevalence is around 56 to 100 cases per 100,000 births [1]; with three types: Ostium primum (20% - 35%), ostium secundum (50% - 70%) and ”sinus venous and coronary sinus” (10% - 15%) [2].
Ostium secundum ASD is accessible to percutaneous closure in 90% of cases; with better results than surgery with a lower complication rate [3].
According to ESC guidelines [4,5], ASD closure is indicated (class I) in case of right ventricle overload and without pulmonary hypertension or in case of paradoxical embolism (class IIa) and contraindicated in Eisenmenger physiology (class III).
The procedure is performed under local anesthesia and TTE or general anesthesia and TEE (for the first procedures and child) through the femoral vein. A stiff guidewire is placed in a pulmonary vein through the ASD, using an MPA catheter, and allows the consecutive rise of the calibration balloon and the sheath that brings the prosthesis until the interatrial septum that it will sandwich under echocardiographic control [6-9].
After the procedure, Aspirin is maintained for 6 months with clinical and echocardiographic control at M1, M3, M6, and M12 [10].
Association of OS-ASD with an azygos continuation of the inferior vena cava, is very rare (<0.1/1000 births) [11] making femoral access impossible [12-14].
Patient information
De-identified patient-specific information: We describe the case of a 32-year-old patient with no history in whom a routine medical examination for asthenia 2 years before the current episode objectified a heart murmur motivating her orientation in cardiology for echocardiography which objectified an ASD OS of 20 mm -21 mm with right cavitary repercussions (RV at 40 mm and RV/LV ratio at 0.9, the right atrium at 20 cm²) and a systolic pulmonary artery pressure at 40 mmHg.
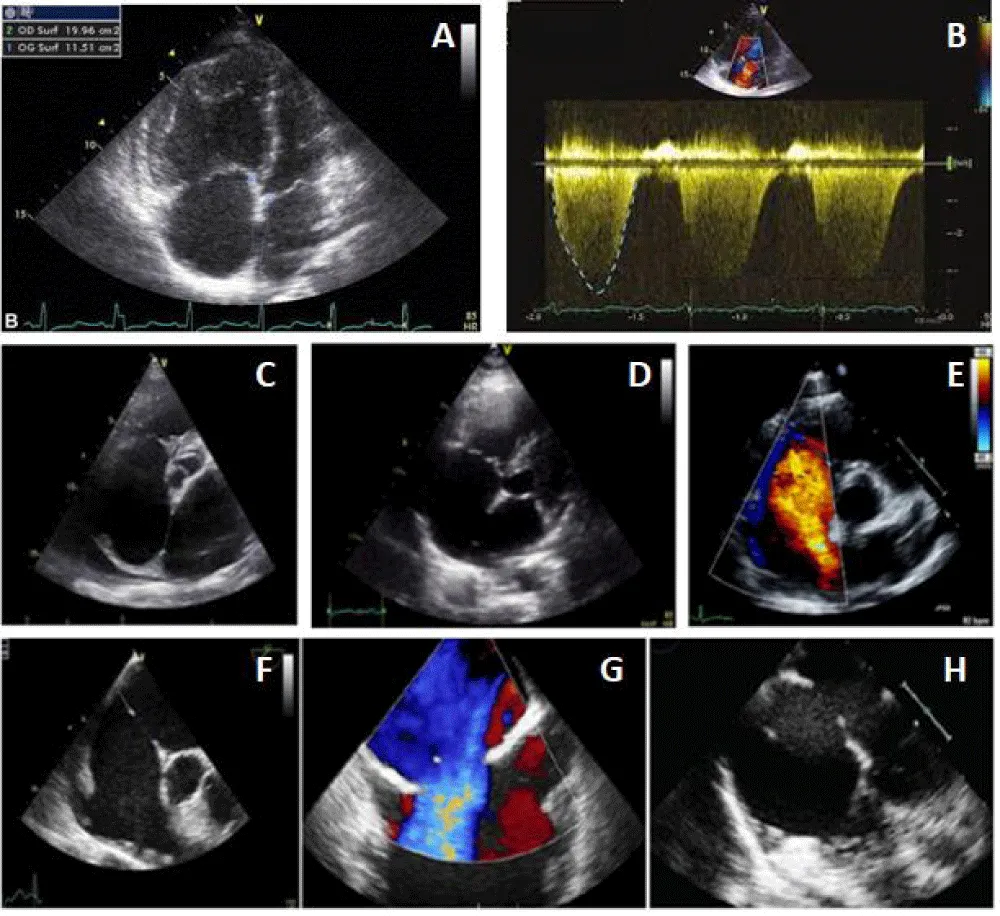
Faced with the desire for pregnancy and with this right cavitary impact and the existence of satisfactory edges (retro-aortic edge 9 mm, posteroinferior edge 10mm, atrioventricular edge 10 mm, upper edge 9 mm, lower cave edge 10 mm, upper cave edge 12 mm) her treating cardiologist decides to entrust her to us for percutaneous closure of this defect (Figure 1).
Figure 1: ASD evaluation before the percutaneous procedure. (A: Right cavities dilatation, B: Tricuspid regurgitation, C-D and E: ASD and edges in TTE, F-G, and H: ASD and edges in TEE).
Clinical findings
We started the procedure under local anesthesia and femoral vein 6Fr access, we introduced an MPA catheter, which drew an unusual path with a loop behind the right atrium to join the superior vena cava and then the right atrium.
Diagnostic assessment
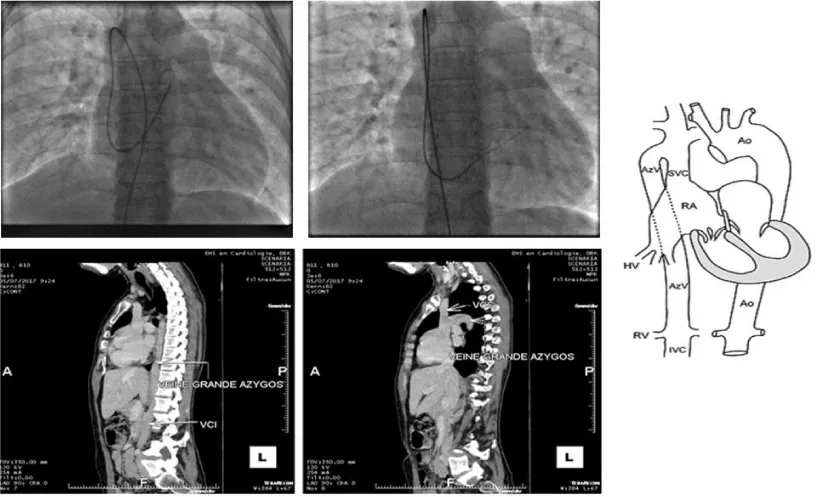
Faced with this trajectory, we suspected an association with an azygos continuation of the inferior vena cava. We interrupted the procedure and requested a CT angiography that confirmed the diagnosis (Figure 2).
Figure 2: Azygos continuation discovered in angiography and CT.
We discussed a surgical closure, which was categorically refused by our patient. We, therefore, performed an ultrasound of the jugular vein in the Trendelenburg position confirming its good caliber.
Therapeutic intervention
We returned to Cath Lab 1 month after, the patient was put under general sedation, intubated, and ventilated, to be able to perform TEE.
We punctured the internal jugular vein in the Trendelenburg position and then 5000 unities of heparin were injected.
We positioned the patient on the catheterization table in the usual position (head up) and we placed, in addition to the usual instrument table on the patient’s right, a second instrument table at the patient’s head forming an L with the first table, in order to be able to handle the delivery system in complete safety.
We started by crossing the ASD via the MPA and we positioned the 0.035’’ stiff guidewire in the upper then lower left pulmonary vein, however each time the angle with which the calibration balloon cross the ASD was unfavorable and brought the 0.035’’ wire back into the right atrium.
We decided then to put the wire in the aorta (through the ASD, then in the LA then LV and finally in the descending aorta as far as possible) in order to increase the support. From then on, the rest of the procedure became simple.
We reintroduced the calibration balloon giving a stretched ASD diameter of 23 mm (measurement by angiography and echocardiography TTE/TEE) fixing our choice on a 26 mm prosthesis which requires a septum height of 40 mm (26 + 2×7) that was exactly our patient’s septum height.
Then, we introduced the 12 Fr sheath from the internal jugular vein and prepared the prosthesis in its chamber as described earlier in this document.
The deployment and release of the prosthesis were done under angiographic and echocardiographic control (TTE/TEE).
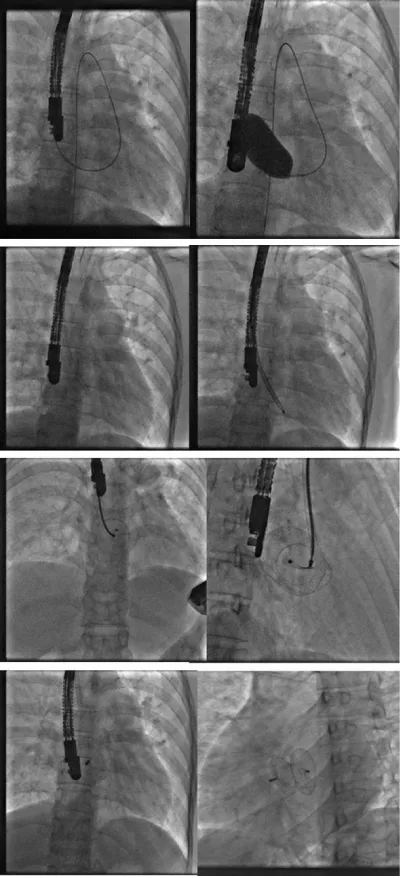
For that, we started by putting the delivery system through the mitral valve in order to increase the support and stabilize the system, then we pushed the prosthesis till the end of its sheath and then we pulled back everything into the left atrium (to avoid trapping the prosthesis in the mitral cords) to deploy the distal disc, brought back into contact with the septum than the proximal disc to thus sandwich the septum between the two discs (Figure 3).
Figure 3: Procedure angiography.
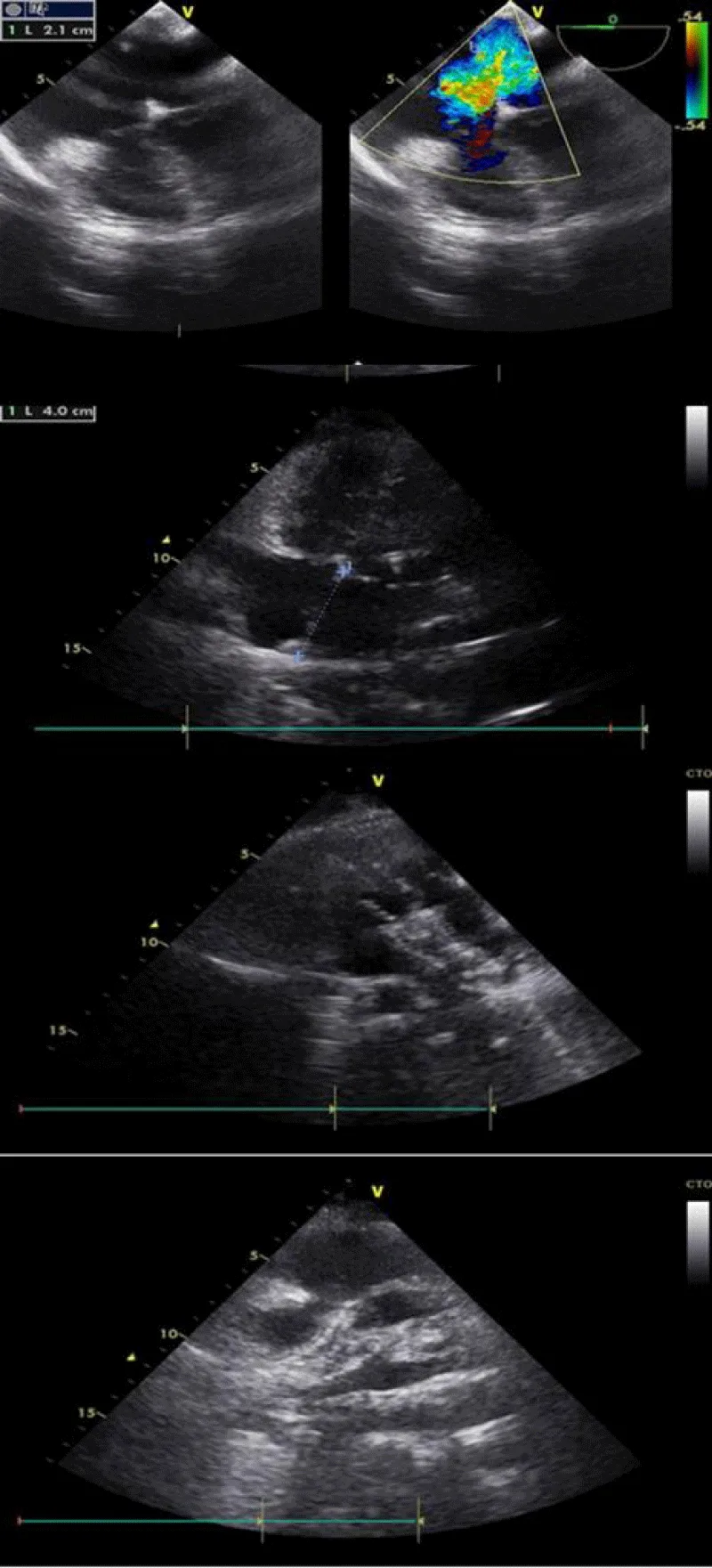
After confirming the correct positioning of the prosthesis and the absence of conflict with the mitral valve, the pulmonary veins, and the superior vena cava, we released successfully the Occluder by anti-clockwise rotation of its rod (Figure 4).
Figure 4: Procedure TTE.
We considered using a femoral closure device (Femoseal©) to close the venous access after removing the delivery system, but having no experience with this device on venous access, we performed simple prolonged manual compression before waking up and extubating the patient. She was then transferred to the recovery room for 6 hours and brought back to the normal hospitalization room the same day.
Follow-up and outcomes
The follow-up was favorable in our patient at the cost of a hematoma at the puncture site and little compression on the brachial plexus with right upper limb anesthesia and paresis, which regressed after 3 days. The patient was discharged three days later under Aspirin for 6 months.
Control 7 days, 1, and 3 months after showing the total disappearance of the neurological disorders in the right upper limb, the absence of residual shunt, and the regression of the right cavity dilation.
At first, we considered the surgical cure that is a reasonable attitude for this patient at low surgical risk, but she refused categorically, and after having considered the feasibility of percutaneous closure we changed our strategy [12-14].
We opted for general anesthesia and intubation in order to facilitate the guidance by TEE.
We were faced with a lack of support when placing the wire in the pulmonary veins as described in the classic ASD closure procedure, which is why we placed it in the aorta, and that gave us good stability to continue successfully the procedure.
We underestimated the risk of complication at the puncture site which could have been avoided by either putting a vascular suture device or more prolonged compression (but which would have required prolonging the patient’s intubation).
The primary take-away lesson of this case: In case of ASD associated with an azygos continuation of the inferior vena cava, the right internal jugular vein remains a reasonable route; it requires discussion and rigorous preparation by the whole team, but also a discussion with the patient.
Learning objectives
1. To know that percutaneous management of the association “ASD and azygos continuation” is possible
2. To know how to perform this particular procedure step by step.
Patient perspective
The patient was very satisfied with the result despite the complication at the puncture site.
Informed consent
The patient consented to the sharing and publishing of her case and procedure images subject to anonymity
Ethics approval and consent to participate
The patient consented to undergo the procedure.
onsent for publication
The patient consented to the sharing and publication of data, images, and results.Availability of data and material
The images presented during this work are available from the corresponding author upon reasonable request.
Authors’ contributions
NZ was responsible for the realization of the percutaneous procedure and participated in the writing of the manuscript.
AB participated in the percutaneous procedure and the realization of echocardiography.
NB participated in the realization of the percutaneous procedure and the writing of the manuscript.
AT participated in the realization of echocardiography and the writing of the manuscript.
MB participated in the follow-up of the patient during and after hospitalization.
All authors read and approved the final manuscript.
- van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011 Nov 15;58(21):2241-7. doi: 10.1016/j.jacc.2011.08.025. PMID: 22078432.
- Naqvi N, McCarthy KP, Ho SY. Anatomy of the atrial septum and interatrial communications. J Thorac Dis. 2018 Sep;10(Suppl 24):S2837-S2847. doi: 10.21037/jtd.2018.02.18. PMID: 30305943; PMCID: PMC6174145.
- Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, Kim D, Kogon B, Oster ME. Transcatheter Versus Surgical Closure of Atrial Septal Defects in Children: A Value Comparison. JACC Cardiovasc Interv. 2016 Jan 11;9(1):79-86. doi: 10.1016/j.jcin.2015.09.028. PMID: 26762915.
- Martin SS, Shapiro EP, Mukherjee M. Atrial septal defects - clinical manifestations, echo assessment, and intervention. Clin Med Insights Cardiol. 2015 Mar 23;8(Suppl 1):93-8. doi: 10.4137/CMC.S15715. PMID: 25861226; PMCID: PMC4373719.
- Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb 11;42(6):563-645. doi: 10.1093/eurheartj/ehaa554. PMID: 32860028.
- Egidy Assenza G, Spinardi L, Mariucci E, Balducci A, Ragni L, Ciuca C, Formigari R, Angeli E, Vornetti G, Gargiulo GD, Donti A. Transcatheter Closure of PFO and ASD: Multimodality Imaging for Patient Selection and Perioperative Guidance. J Cardiovasc Dev Dis. 2021 Jul 3;8(7):78. doi: 10.3390/jcdd8070078. PMID: 34357321; PMCID: PMC8306204.
- Chan KC, Godman MJ, Walsh K, Wilson N, Redington A, Gibbs JL. Transcatheter closure of atrial septal defect and interatrial communications with a new self expanding nitinol double disc device (Amplatzer septal occluder): multicentre UK experience. Heart. 1999 Sep;82(3):300-6. doi: 10.1136/hrt.82.3.300. PMID: 10455079; PMCID: PMC1729188.
- Harper RW, Mottram PM, McGaw DJ. Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc Interv. 2002 Dec;57(4):508-24. doi: 10.1002/ccd.10353. PMID: 12455087.
- ASD_Closing-procedure.pdf (iwate-shd.jp)
- Yang MC, Wu JR. Recent review of transcatheter closure of atrial septal defect. Kaohsiung J Med Sci. 2018 Jul;34(7):363-369. doi: 10.1016/j.kjms.2018.05.001. Epub 2018 May 30. PMID: 30063008.
- Boffa GM, Chioin R, Stritoni P, Daliento L, Congedo E, Sperandeo V, Scognamiglio R. Continuazione azigos o emiazigos della vena cava inferiore. Studio angiografico di 10 casi [Azygos or hemiazygos continuation of the inferior vena cava. An angiographic findings of 10 cases (author's transl)]. G Ital Cardiol. 1980;10(8):1063-8. Italian. PMID: 7461348.
- Seshagiri RD, Patnaik AN, Srinivas B. Percutaneous closure of atrial septal defect via transjugular approach with Blockaid device in a patient with interrupted inferior vena cava. Cardiovasc Interv Ther. 2013 Jan;28(1):63-5. doi: 10.1007/s12928-012-0113-4. Epub 2012 Jul 19. Erratum in: Cardiovasc Interv Ther. 2013 Jan;28(1):137. PMID: 22810920.
- Oliveira EC, Moura MAG, Almeida JA, Ribeiro ALP, Nascimento BR. Percutaneous closure of ostium secundum atrial septal defect using left internal jugular vein access in a child with situs inversus and absence of inferior caval vein. Cardiol Young. 2019 Oct;29(10):1310-1312. doi: 10.1017/S1047951119002099. Epub 2019 Sep 2. PMID: 31475660.
- Ozdemir E, Emren S V, Eren N K, Nazli C, Tokac M. Transjugular closure of ASD in a patient with interrupted inferior vena cava. Int J Cardiovasc Acad. 2018; 4: 15-18.