More Information
Submitted: September 13, 2023 | Approved: September 26, 2023 | Published: September 27, 2023
How to cite this article: Del Río IJ, Medinilla EV, Albarrán AA, Rubio Alonso MA, Peinado AA. Conduit quality control protocol in CABG. J Cardiol Cardiovasc Med. 2023; 8: 122-129.
DOI: 10.29328/journal.jccm.1001164
Copyright License: © 2023 Del Río IJ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: CABG; Quality control; TTFM; Epiaortic echography; Quality protocol; Conduit patency
Conduit quality control protocol in CABG
Ignacio Juárez Del Río*, Enrique Villagrán Medinilla, Ali Ayaon Albarrán, Miguel Angel Rubio Alonso and Angel Aroca Peinado
Cardiovascular Surgery Departement, Hospital, Universitario La Paz, Spain
*Address for Correspondence: Ignacio Juárez Del Río, MD, Cardiovascular Surgery Departement, Hospital, Universitario La Paz, Spain, Email: [email protected]
Cardiac revascularization surgery has a long history. Its results and safety are well known. Nonetheless, the long-term patency rate of certain grafts used in cardiac revascularization is non-optimal, and CABG is associated with a risk of cerebrovascular stroke due to aortic manipulation. We have developed a simple control quality protocol of the anastomosis performed in CABG, aiming to improve the long-term patency of certain grafts used in cardiac revascularization surgery and reduce the risk of cerebrovascular stroke in those patients.
Cardiac revascularization surgery is a treatment with more than 60 years of experience (the first bypass graft was performed in the 1960s) [1] and is a surgical technique with consolidated results: when we compare cardiac revascularization surgery to medical treatment of ischemic cardiopathy, 5 year’s, 7 year’s and 10 year’s mortality is inferior in the cardiac revascularization surgery group [2].
Besides, surgical treatment of ischemic cardiopathy, when compared to percutaneous treatment, allows a more complete quality of cardiac revascularization, without differences in terms of mortality, heart infarction, or cerebral stroke [3].
We also have to consider that when we compare percutaneous cardiac revascularization to medical treatment of ischemic cardiopathy in patients with stable angina, no significant differences are observed in terms of mortality risk or heart infarction [4]. Also, cardiac revascularization surgery is a surgical technique with a low rate of complications (for instance in patients with complex lesions in their coronaries arteries, patients treated with percutaneous revascularization present 5 years after the intervention a higher rate of complications than those treated with surgery) [5]. This study proved that after 5 years, death rate, neurological stroke or cardiac infarction, as well as the need for re-revascularization was statistically superior in the group of patients with percutaneous cardiac revascularization.
Due to these facts, cardiac revascularization surgery is recommended in patients with “unprotected” left main coronary lesions (that is to say in patients with a > 50% stenosis of the left main coronary artery without bypass grafts), in patients with multivessel coronary arteries disease, or with a left ventricle dysfunction and a SYNTAX score > 22. In addition, in patients with diabetes mellitus and multivessel disease, surgical revascularization should be the first revascularization choice regardless of the SYNTAX score [6].
All these facts make cardiac revascularization surgery the most frequent cardiac surgery worldwide [1], although and despite everything, is a surgery not without risk, among the most frequent: is cerebral stroke due to manipulation of the aorta since the load of vascular damage in these patients is usually widespread. This risk could be reduced by the use of TTFM and periaortic echography, which allows to asses of the calcification of the aortic wall, enabling to choose of a healthy aortic wall as a clamping site, or a site to perform the proximal anastomosis in CABG.
On the other hand, the advent and the heyday of percutaneous revascularization techniques have changed the profile of the patient that faces the cardiac revascularization surgery, being more fragile, with more calcified arteries, and with a greater number of stents previously implanted, which hinders, even more, the surgical technique [7].
Thus, cardiac surgeons nowadays face a major challenge: to realize quality anastomosis in coronary arteries with complex vascular walls, they have to avoid also the risk of cerebrovascular complications associated with the manipulation of the ascending aorta in these patients, for what it’s imposed an evaluation of the result of the anastomosis performed in CABG.
Furthermore, it has been shown that the patency of the graft performed in cardiac revascularization surgery depends on the graft chosen, and on the coronary artery that we want to revascularize [8].
The next table (Table 1) gathers the patency rate 10 years after surgery, of the different grafts in the function of the coronary artery in which they are anastomosed.
| Table 1: Patency rate 10 years after surgery of the different grafts. | |||
| Anterior descending artery | Circumflex artery | Right coronary artery | |
| Left internal mammary artery | 97% | 89% | 84% |
| Right internal mammary artery | 91,9% | 90,1% | 83,1% |
| Radial artery | 72% - 84% | 72% - 84% | 72% - 84% |
| Great saphenous vein | 50% - 60% | 50% - 60% | 50% - 60% |
Furthermore, patency rates of both venous [9] and arterial [10] grafts depend on other factors such as the diameter of the graft, and the severity of the stenosis in the coronary artery.
As the results show, the graft with the worst patency rate is the great saphenous vein, which has led the pharmaceutical industry to research intraoperative preservation solutions for the graft [11], and the development of external stents to protect these grafts, since they mitigate the vascular wall remodeling of the saphenous vein, they reduce the intimal hyperplasia of the wall of the vessel, as well as the lumen irregularity, 4 to 5 years after surgery [12].
All these efforts to improve the long-term patency rate of the grafts used in CABG should be endorsed by the surgical team in charge of the procedure, who are committed to performing the best possible anastomosis in every circumstance, this is why quality control of each anastomosis performed in CABG should be carried out routinely, and that’s the reason why we present a protocol, based in the measure of flow and in the epiaortic echography, that allows a simple quality control.
Quality control of the anastomosis performed in cardiac revascularization surgery was proposed in the 1960s [13]. The first flow measurers were based on electromagnetic technology but were soon discarded because of their multiple limitations.
In the 1980’s the first flow measures based on the Doppler principle appeared, which meant a revolution in flow measurement, and the development of beating heart cardiac revascularization surgery techniques in the 1990s, which generated the need to prove that the anastomosis performed were functional, sparked the heyday of flow measurement.
It’s also at this time when the technology that enables the measurement of the “TTFM” (Transit Time Flow Meter) appears. The TTFM is a simple reproducible method and is the system chosen nowadays to perform flow measurements through an anastomosis. The difference between TTFM and Doppler, it’s that the probes that measure TTFM include 2 piezoelectric crystals and a metallic reflector in the opposite site, while the Doppler probes include a single piezoelectric crystal.
We have developed a very simple 3 steps protocol, with the aim to assess the quality of the anastomosis performed in CABG. To perform these protocols we use the Medistim MiraQ® system, which allows to measurement of the flow through the anastomosis performed, by TTFM measurement, and also to perform epicardial ethnographies with HFUS (intraoperative High Frequency UltraSound imaging system).
The Medistim MiraQ® system is an easy-to-use system, it’s composed of a console, that allows the measurement of flow through and anastomosis using the measuring system of TTFM, and also allows to perform epicardic echographies (Figures 1-3).
Figure 1: Console of the Medistim MiraQ®.
Figure 2: Flow measurement probe.
Figure 3: Epicardic echography probe.
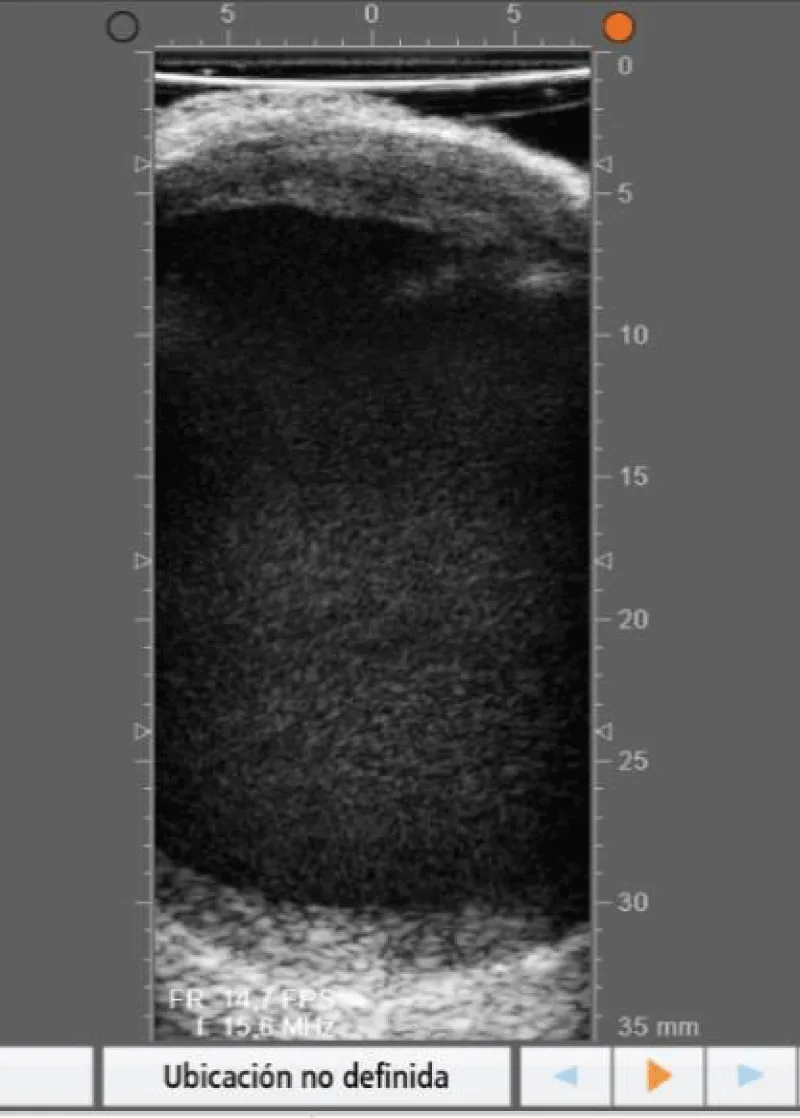
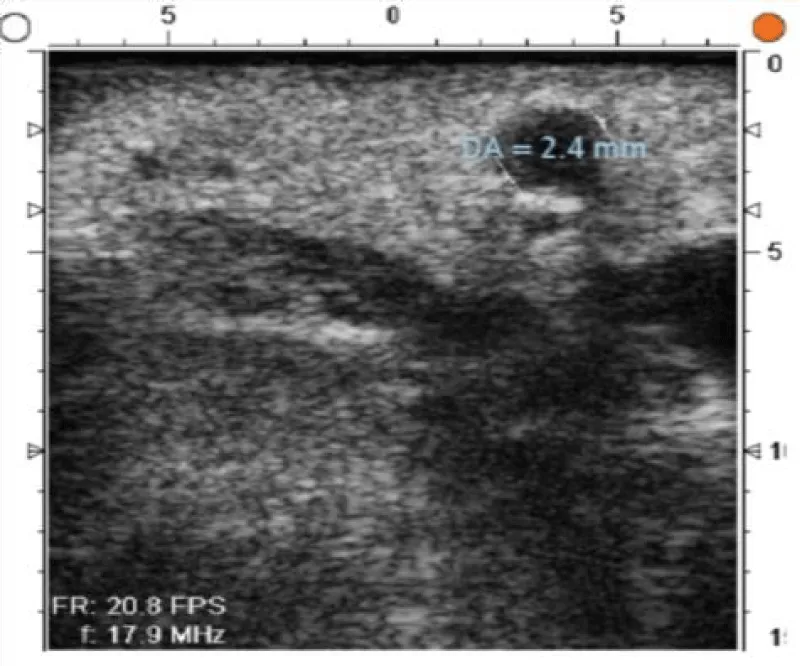
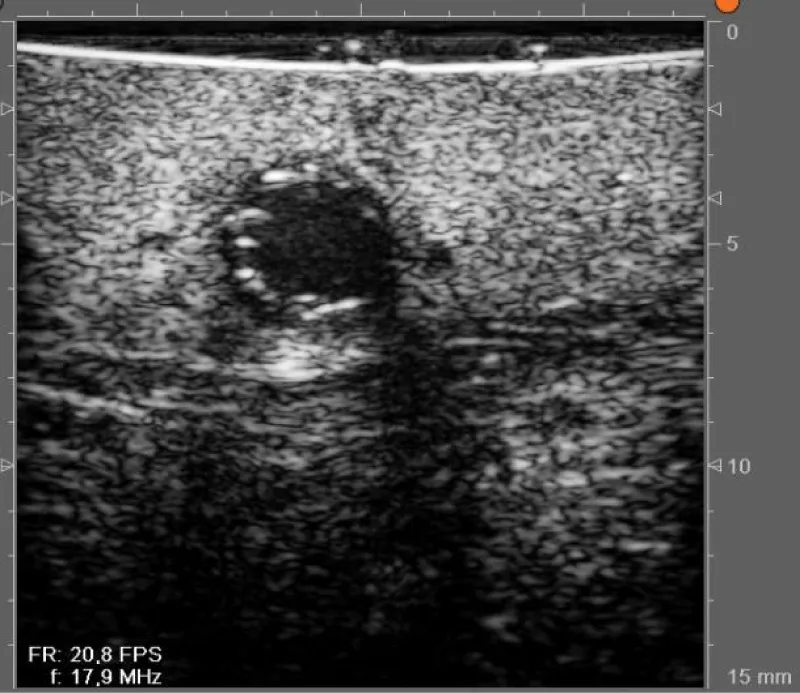
The goal of the first step of the protocol is to perform a global evaluation of the vascular damage of the patient, paying special attention to the state of the aortic wall (where cannulation, cross-clamping site in case of surgery with extracorporeal circulation, and lateral clamping to perform the proximal anastomosis, need to be chosen) (Figures 4,5) and also to locate the ideal place to perform the anastomosis between the graft and the coronary artery (Figures 6-8).
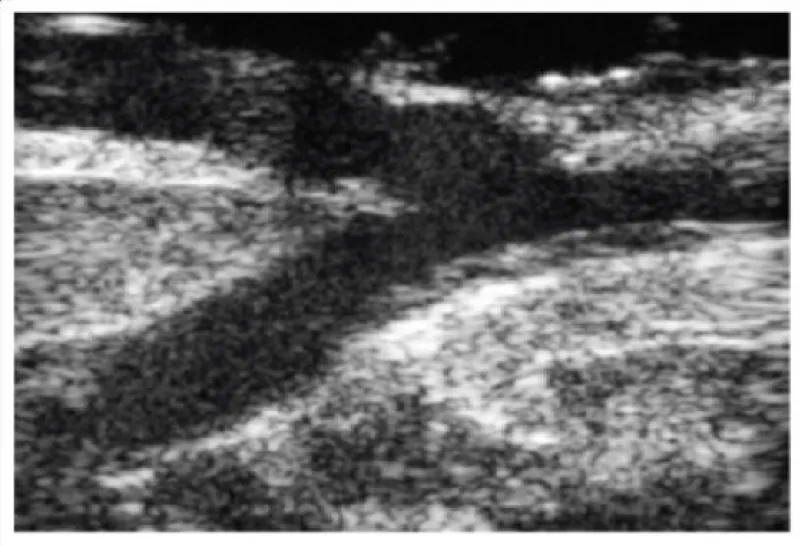
Figure 4: Echography showing an aortic with an intact anterior wall.
Figure 5: Echography showing an aortic wall with a plaque in the anterior wall.
Figure 6: Anterior descending coronary artery with 2,4mm diameter and an intact arterial wall.
Figure 7: Anterior descending coronary artery with a plaque in the posterior wall.
Figure 8: Coronary artery with a stent.
The second step of the protocol consists of an early evaluation of the result of the anastomosis performed.
The last step of the protocol aims to perform a final evaluation of the result of the anastomosis performed.
Therefore, our protocol allows an evaluation of the flow through the anastomosis performed, and also the detection of ateromatosis plaques in the ascending aorta wall, two items that are part of the ESC/EACTS guidelines of cardiac revascularization therapy [14].
This echographic evaluation of the state of the walls of the ascending aorta and the coronaries arteries can be key in the execution of the surgical strategy preconceived by the cardiac surgeon, since the most recent publication, the echografic evaluation of the arterial walls states changed the surgical plan in 25% of the cases of these study [15].
To standardize the use of this evaluation, we have developed a 3-step protocol, that we resume in Table 2.
| Table 2: The 3 steps of the protocol. | |
| When it’s performed? | Objective |
| After pericardial opening | Epiaortic echography evaluation of the state of the ascending aortic and the coronary arteries walls. |
| When the distal anastomosis is performed | Early evaluation of the result of the anastomosis measuring the flow through the graft. It can be completed with echography of the anastomosis in case of doubts. |
| After administration of protamine | Final evaluation of the result of the anastomosis measuring the flow through the graft. It can be completed with echography of the anastomosis in case of doubts. |
The first step
We realized it prior to the beginning of the extracorporeal circulation, and it’s based on the use of epicardic echography to:
- Precisely locate the course of the coronary arteries (this is really important in patients with an important amount of epicardic fat, in reoperated patients that present an important epicarditis, and in patients with CABG).
- Asses precisely the state of the entire arterial wall of the coronaries arteries (it allows the evaluation of the state of the wall of the coronaries arteries, which it’s impossible to perform precisely with a fingertip palpation).
- Asses precisely the state of the wall of the ascending aorta, so that will be able to choose the ideal location to perform the anastomosis between the graft and the aorta, and the cross-clamping site.
These steps of the protocol is an indispensable part of the protocol and can result in the modification of the surgical strategy that was planned before the beginning of the surgery, since the detection of an aortic wall with multiple calcification sites makes a safe cross-clamping and performing quality proximal anastomosis, could make the surgeon rethink his surgical strategy, favoring in those cases to perform an offpump CABG, with sequential or “T” disposition anastomosis.
The correct acquisition of echographic images and precise technical details such as the setting of the echographic probe in the sterile sheath (avoiding rests of air inside the sheath) (Figure 9), and the correct angulation of the echographic probe when placed in the epicardial surface (Figure 10).
Figure 9: Correct setting of the echographic probe in the sterile sheath.
Figure 10: Correct angulation of the echographic probe when placed on the epicardial surface.
The time requested to perform the first step of the protocol usually consumes less than a minute when the results are satisfactory. When results are unsatisfactory, we recommend redoing the anastomosis, as we are in an early stage of the surgery, and this will be time-saving if we pursue surgery and the result of the anastomosis is not satisfactory.
The second step
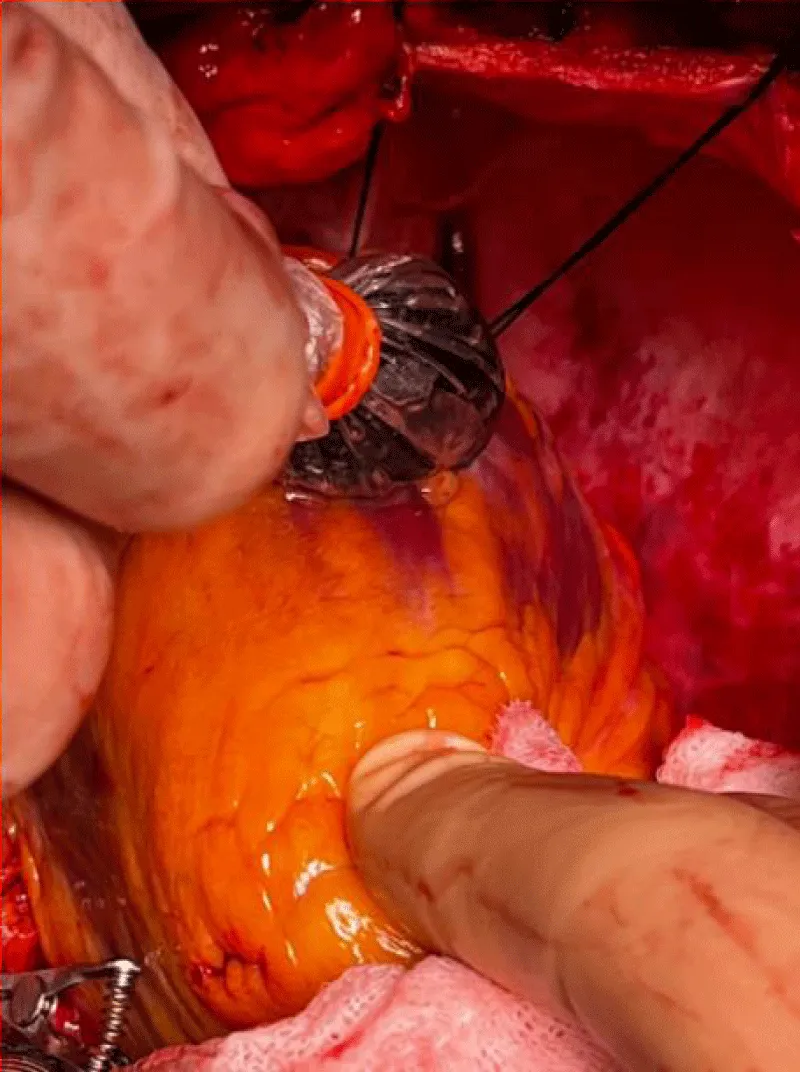
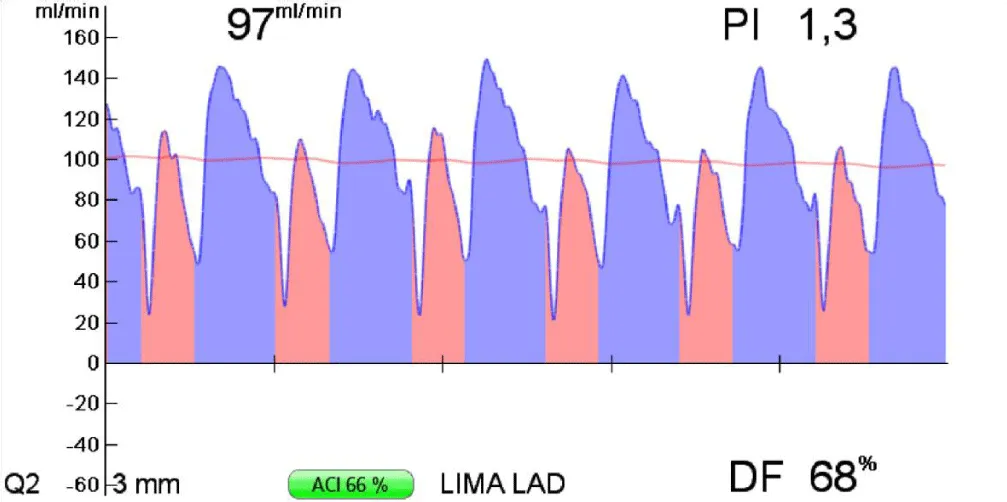
It’s performed after the realization of the anastomosis between the graft and the coronary artery. It allows an early evaluation of the result of the anastomosis, and it’s based on the measurement of flow through the graft anastomosed to the coronary artery Figure 11.
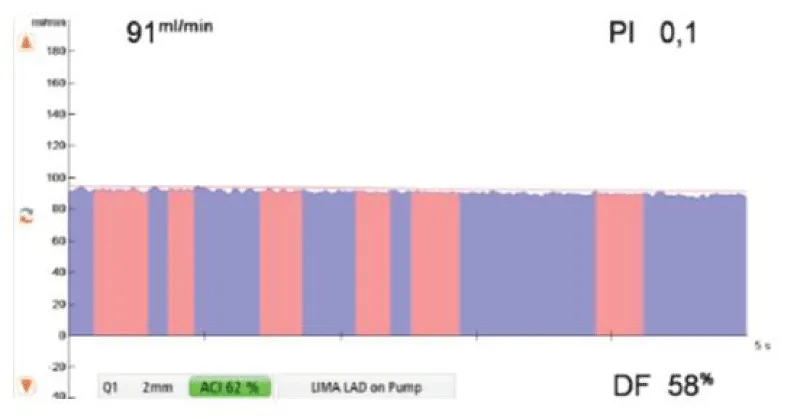
Figure 11: Example of flow measurement after realization of an anastomosis between the left internal mammary artery and the left anterior descending coronary artery, during extracorporeal circulation [16].
The parameters that evaluate the quality of the anastomosis are the Pulsatility Index (PI), the Diastolic Filling (DF), and the flow volume (measured in ml/min). During extracorporeal circulation, from these parameters, the only one that has to be considered to evaluate the quality of the anastomosis is the PI, and a PI inferior to 0,4 reflects a good quality anastomosis.
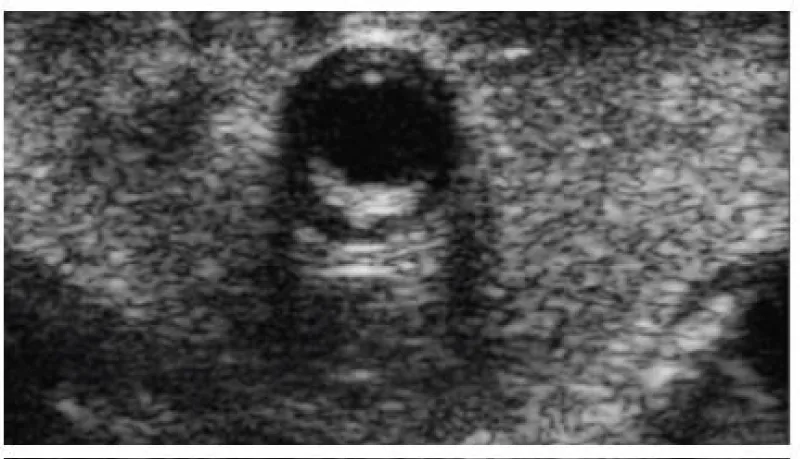
In case of doubt about the quality of the anastomosis (due to technical challenges during the realization of the anastomosis, or obtainment of nonideal PI values), we can assess the result of the anastomosis with the epicardic echography (Figures 12,13):
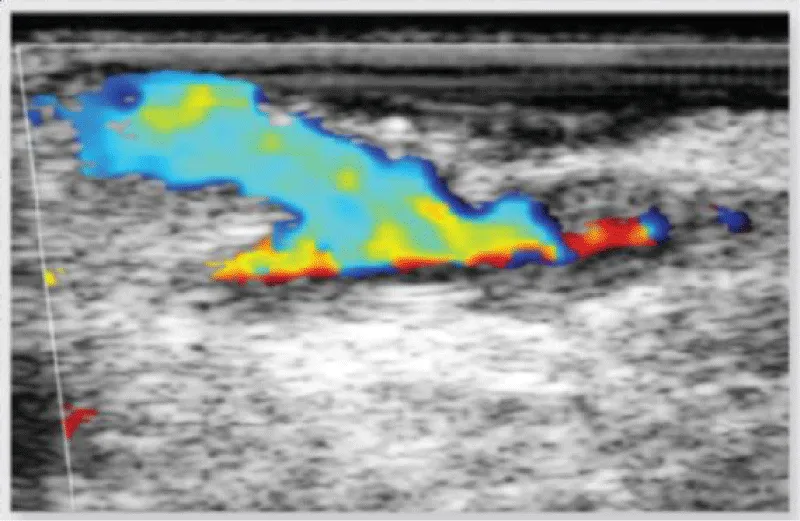
Figure 12: Echographic evaluation of an anastomosis between the left internal mammary artery and the left anterior descending artery.
Figure 13: Doppler echographic evaluation of an anastomosis between the left internal mammary artery and the left anterior descending artery.
To obtain flow measures that are representative of the state of the anastomosis requires some simple conditions of use of the device:
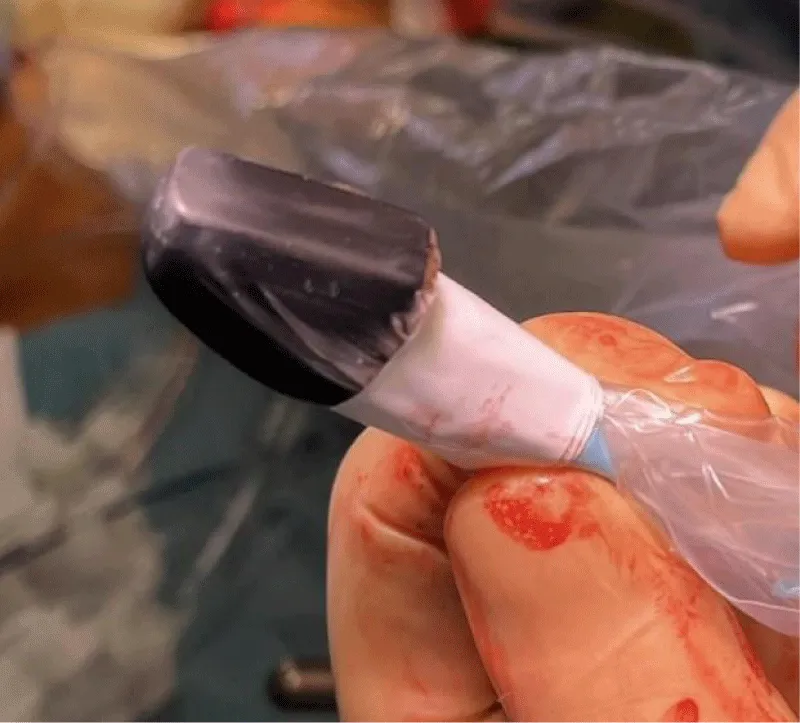
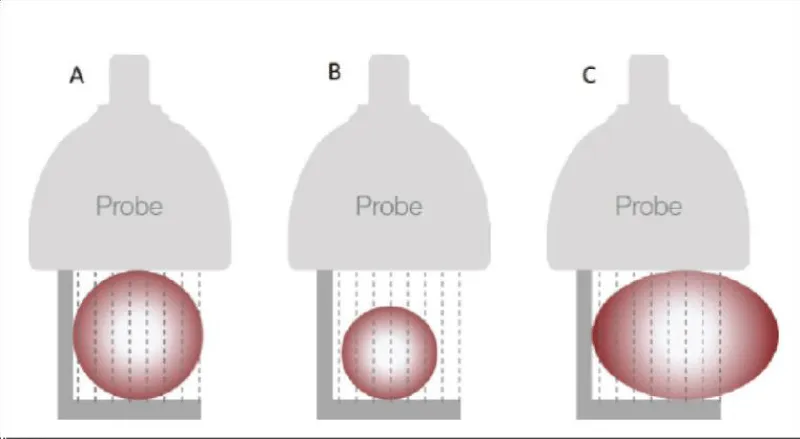
- Choose the adequate size of the probe, depending on the diameter of the graft (Figure 14).
Figure 14: The right choice of the size of the probe (images provided by Medistim®).
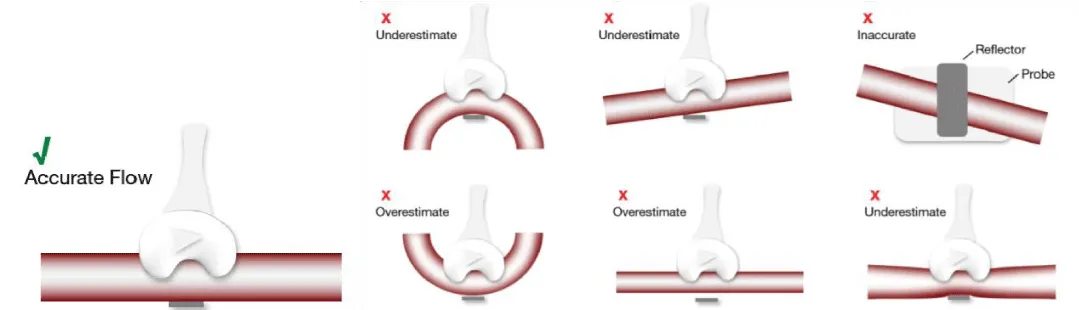
- Correct placement of the probe around the graft, and in case of utilization of pediculated grafts, dissect the tissue around the graft for a correct placement (Figure 15)
Figure 15: Correct placement of the probe vs. examples of inaccurate placement of the probe (images provided by Medistim®).
- Apply echographic gel to the probe
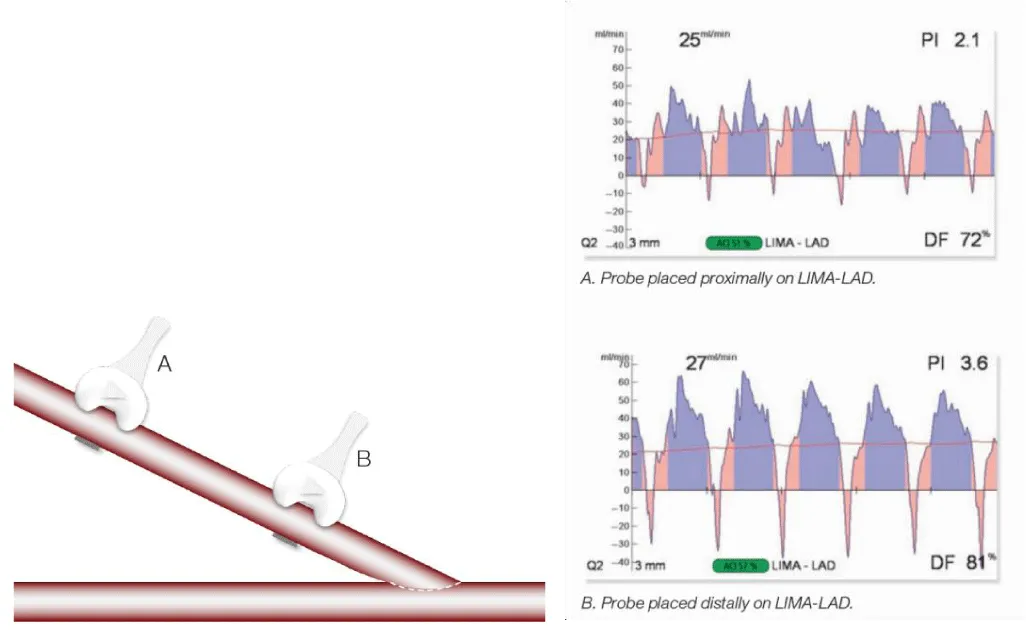
- Perform the measurement close to the anastomosis (1 cm) that we want to evaluate (Figure 16).
Figure 16: Differences in records of flow measurement depending on the placement of the probe (images provided by Medistim®).
The parameters that reflect the patency of these anastomosis at this stage of the protocol (with extracorporeal circulation and aortic cross-clamp) are collected in Table 3.
| Table 3: Parameters of anastomosis patency in the second step of the protocol [17]. | |||
| Flow | PI | DF | Backflow |
|
Less than 0.4 | Non appliable at this stage | Non appliable at this stage |
The time requested to perform the second step of the protocol usually consumes less than a minute when the results are satisfactory. When results are unsatisfactory, we recommend to evaluate closely the proximal anastomosis (the evaluation with epiaortic anastomosis can be interesting) and redoing the proximal anastomosis if necessary.
The third step
It’s performed once the extracorporeal circulation is over, the protamine is administered, and previous to sternal closure, which gives information in the closest fisiological hemodynamic state. It’s also based on the use of flow measurement through the graft, and in this stage, the ideal value of PI has to be less than 5, the diastolic filling greater than 70% and the flow volume greater than 15 ml/min. (Figure 17).
Figure 17: Flow measurement through the left internal mammary artery, anastomosed to the left anterior descending coronary artery.
Previous to performing the last flow measurement we recommend performing a visual inspection of the final disposition of the grafts to discard the presence of graft torsions along its course, which can be caused when the graft presents an excessive length and can jeopardize the flow through the graft.
When in the presence of an aortic wall with plaques and calcifications, an echographic evaluation of the result of the proximal anastomosis can be interesting and could rule out the presence of complications in those anastomosis.
At this point of the surgery, and if the values obtained with the flow measurement system are ideal, the surgeon can be certain that the anastomosis he has performed is permeable, and of good quality, therefore it’s recommended to terminate the procedure at this point.
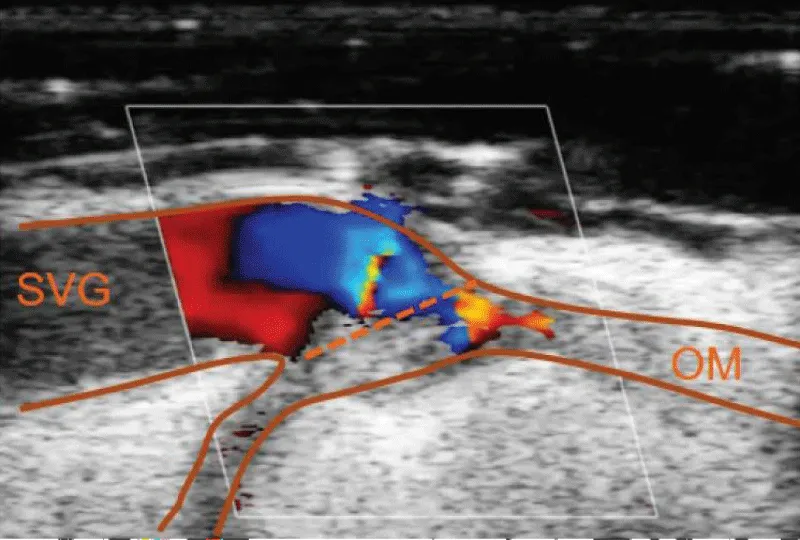
In case of obtaining dissenting values in comparison to the ideal measurement parameters or in case of doubt of the quality of the anastomosis performed, the epicardic echography presents as a valuable tool to provide a visual evaluation of the anastomosis, and sole the doubts (Figure 18).
Figure 18: Image of verification of an anastomosis between saphenous vein and marginal obtuse coronary artery, with Doppler echography, showing a decrease in the flow through the anastomosis due to the presence of a flap of the venous graft.
The parameters reflecting the patency of the anastomosis performed during this stage of the protocol (once the extracorporeal circulation is over) are reflected in Table 4.
| Table 4: Parameters of the anastomosis patency in the third step of the protocol [17]. | |||
| Flow | PI | DF | Backflow |
|
Less than 5 (ideally less than 3) | Greater than 50% in right side Greater than 60% - 70% in left side |
Less than 3% |
The use of the flow measurement and epicardic echography devices in CABG provides us with important information about the state of the anastomosis performed, but also about the state of the vessels of the patient, and the flow running through these vessels. All this information could be extrapolated a other cardiac surgery interventions, giving important information to the surgeon. In our work group, we have detected some potential utilization of this technology which we detail below:
- In aortic arch replacement surgery with reimplantation of the supra-aortic trunks: it allows flow measurement through the supra-aortic trunks
- In redo’s when the first CABG was performed, it allows to precisely locate the course of the previous grafts (Figure 19).
Figure 19: Location with epicardic echography of the course of an internal mammary artery anastomosed to the left anterior descending artery, in a patient reoperated, in whose first intervention a cardiac surgical revascularization was performed.
- In redo’s when myocardial revascularization has to be performed in the present surgery, or in patients with an important amount of pericardial fat, it allows to precisely locate the course of the coronary arteries.
- When an aortic aneurysm is suspected, it allows an accurate in situ measurement of the aortic diameter, so that we can assess the suitability of an ascending aorta replacement.
- Dissection of the internal mammary artery allows to confirm the patency of the graft in cases of important spasmodization of the artery, or in case of suspicion of dissection of the vessel wall.
- Also, previous dissection of the internal mammary artery, allows the location of the artery with the epicardial echography and confirms the presence of adequate flow with Doppler echography prior to the graft extraction.
- In case of doubt if the coronary vessel is a vein or an artery.
CABG is a surgical technique with a great history and excellent short-term results, but its thin point concentrates on the long-term patency rates of certain grafts. To improve these patency rates, cardiac surgeons should perform the best technically feasible anastomosis and assess the quality of that anastomosis.
With this goal, we have developed a simple protocol, made of 3 steps, that allows the surgeon, first to evaluate the quality of the coronary arteries and of the aorta of the patient, second to asses precociously the quality of the anastomosis performed, allowing him to an early decision taking when the results obtained aren’t satisfying, and third a final evaluation of the result and the patency of the anastomosis performed, that can be simply applied without adding extra time to the surgery.
Perform a meticulous CABG is nowadays an increasingly important factor, due to the advent and heyday of the percutaneous revascularization techniques that have decreased the number of patients candidates for CABG, and have changed the profile of these patients, being nowadays more fragile and with more comorbidities, and even all these setbacks, CABG continues to be more effective than percutaneous revascularization techniques. For instance, patients with diabetes are more likely to have main steam disease or multivessel disease, involving smaller vessels [18].
All these circumstances force the cardiac surgeon to perform anastomosis in more hostile territories, like coronary arteries with severe wall calcifications, or coronary arteries with previous stent implantations, so the location of an ideal place to perform an anastomosis is a critical step for the success of the surgery.
Since the patients that have to face cardiac revascularization surgery are nowadays more fragile, cardiac surgeons must perform a greater number of bypass grafts, but with the certainty that those are effective. The evaluation of the coronary anatomy with the epicardic echography and the assessment of the flow through the anastomosis has demonstrated to change the surgical preoperatory strategy in 25% of the cases, which could be very important for fragile patients, in which we could reduce the surgical insult, avoiding the performance of anastomosis in important damaged coronary arteries, where surely the result of the anastomosis won’t be adequate, and favoring in these cases a hybrid revascularization approach, improving the patient evolution. We can’t forget that CABG entails an important risk of cerebrovascular stroke associated with the ascending aorta manipulation necessary for the performance of proximal anastomosis, or for aortic cross-clamping in cases with extracorporeal circulation surgery, because patients with coronary lesions usually present disseminated vascular damage, and present aortic calcified walls, for this reason, the evaluation of the wall state of the aorta with the epicardic echography is a simple technique within range of the cardiac surgeons, that could decrease the risk of cerebrovascular strokes in a patient undergoing cardiac revascularization surgery, as periaortic wall evaluation with periaortic echography enables to locate regions of the healthy aortic wall, where the risk of calcium embolus associated to aortic plaque rupture associated to aortic cross-clamping, or performance of proximal anastomoses, is lower.
We have developed a simple protocol to assess the quality of the anastomosis performed in CABG, with and without extracorporeal circulation, allowing the ideal location to perform the anastomosis in the coronary artery first, and a second time to evaluate the quality of the anastomosis performed.
We hope the implementation of the control quality protocol of the anastomosis performed in CABG assures the performance of quality bypass grafts, that ensure the excellent short-term results of the cardiac revascularization surgery, and allows to increase in the long-term patency rate of certain grafts used in CABG, raising then our patients survival.
This protocol is very simple and easily reproducible, and it’s based on an easy technology simple to obtain in any hospital.
Ethical considerations
All the surgical photos/Figures were obtained during programmed surgery, prior authorization of patients, and the patent in the Informed Consent Document was signed preoperatively by the patient and the surgeon.
- Mack MJ, Squiers JJ, Lytle BW, DiMaio JM, Mohr FW. Myocardial Revascularization Surgery: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol. 2021 Jul 27;78(4):365-383. doi: 10.1016/j.jacc.2021.04.099. Erratum in: J Am Coll Cardiol. 2022 Oct 18;80(16):1582-1583. PMID: 34294272.
- Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, Davis K, Killip T, Passamani E, Norris R, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994 Aug 27;344(8922):563-70. doi: 10.1016/s0140-6736(94)91963-1. Erratum in: Lancet 1994 Nov 19;344(8934):1446. PMID: 7914958.
- Sedlis SP, Morrison DA, Lorin JD, Esposito R, Sethi G, Sacks J, Henderson W, Grover F, Ramanathan KB, Weiman D, Saucedo J, Antakli T, Paramesh V, Pett S, Vernon S, Birjiniuk V, Welt F, Krucoff M, Wolfe W, Lucke JC, Mediratta S, Booth D, Murphy E, Ward H, Miller L, Kiesz S, Barbiere C, Lewis D; Investigators of the Dept. of Veterans Affairs Cooperative Study #385, the Angina With Extremely Serious Operative Mortality Evaluation (AWESOME). Percutaneous coronary intervention versus coronary bypass graft surgery for diabetic patients with unstable angina and risk factors for adverse outcomes with bypass: outcome of diabetic patients in the AWESOME randomized trial and registry. J Am Coll Cardiol. 2002 Nov 6;40(9):1555-66. doi: 10.1016/s0735-1097(02)02346-x. PMID: 12427406.
- Henderson RA, Pocock SJ, Clayton TC, Knight R, Fox KA, Julian DG, Chamberlain DA; Second Randomized Intervention Treatment of Angina (RITA-2) Trial Participants. Seven-year outcome in the RITA-2 trial: coronary angioplasty versus medical therapy. J Am Coll Cardiol. 2003 Oct 1;42(7):1161-70. doi: 10.1016/s0735-1097(03)00951-3. PMID: 14522473.
- Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013 Feb 23;381(9867):629-38. doi: 10.1016/S0140-6736(13)60141-5. PMID: 23439102.
- Deb S, Wijeysundera HC, Ko DT, Tsubota H, Hill S, Fremes SE. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: a systematic review. JAMA. 2013 Nov 20;310(19):2086-95. doi: 10.1001/jama.2013.281718. PMID: 24240936.
- Thielmann M, Neuhäuser M, Knipp S, Kottenberg-Assenmacher E, Marr A, Pizanis N, Hartmann M, Kamler M, Massoudy P, Jakob H. Prognostic impact of previous percutaneous coronary intervention in patients with diabetes mellitus and triple-vessel disease undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007 Aug;134(2):470-6. doi: 10.1016/j.jtcvs.2007.04.019. PMID: 17662792.
- Martínez-González B, Reyes-Hernández CG, Quiroga-Garza A, Rodríguez-Rodríguez VE, Esparza-Hernández CN, Elizondo-Omaña RE, Guzmán-López S. Conduits Used in Coronary Artery Bypass Grafting: A Review of Morphological Studies. Ann Thorac Cardiovasc Surg. 2017 Apr 20;23(2):55-65. doi: 10.5761/atcs.ra.16-00178. Epub 2017 Feb 14. PMID: 28202895; PMCID: PMC5422630.
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998 Mar 10;97(9):916-31. doi: 10.1161/01.cir.97.9.916. PMID: 9521341.
- Taggart DP. Current status of arterial grafts for coronary artery bypass grafting. Ann Cardiothorac Surg. 2013 Jul;2(4):427-30. doi: 10.3978/j.issn.2225-319X.2013.07.21. PMID: 23977618; PMCID: PMC3741887.
- Woodward LC, Antoniades C, Taggart DP. Intraoperative Vein Graft Preservation: What Is the Solution? Ann Thorac Surg. 2016 Nov;102(5):1736-1746. doi: 10.1016/j.athoracsur.2016.05.097. Epub 2016 Sep 10. PMID: 27624295.
- Taggart DP, Webb CM, Desouza A, Yadav R, Channon KM, De Robertis F, Di Mario C. Long-term performance of an external stent for saphenous vein grafts: the VEST IV trial. J Cardiothorac Surg. 2018 Nov 19;13(1):117. doi: 10.1186/s13019-018-0803-9. PMID: 30453984; PMCID: PMC6245530.
- D'Ancona D. Intraoperative graft patency verification: Should you trust your fingertips?. Intraoperative Flow Measurement in Coronary Artery Surgery. 2000; 119.
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165. doi: 10.1093/eurheartj/ehy394. Erratum in: Eur Heart J. 2019 Oct 1;40(37):3096. PMID: 30165437.
- Taggart DP, Thuijs DJFM, Di Giammarco G, Puskas JD, Wendt D, Trachiotis GD, Kieser TM, Kappetein AP, Head SJ. Intraoperative transit-time flow measurement and high-frequency ultrasound assessment in coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2020 Apr;159(4):1283-1292.e2. doi: 10.1016/j.jtcvs.2019.05.087. Epub 2019 Aug 22. PMID: 31685277.
- Kieser TM. Graft quality verification in coronary artery bypass graft surgery: how, when and why? Curr Opin Cardiol. 2017 Nov;32(6):722-736. doi: 10.1097/HCO.0000000000000452. Erratum in: Curr Opin Cardiol. 2018 Jan;33(1):121. PMID: 28806185.
- Thuijs DJFM, Bekker MWA, Taggart DP, Kappetein AP, Kieser TM, Wendt D, Di Giammarco G, Trachiotis GD, Puskas JD, Head SJ. Improving coronary artery bypass grafting: a systematic review and meta-analysis on the impact of adopting transit-time flow measurement. Eur J Cardiothorac Surg. 2019 Oct 1;56(4):654-663. doi: 10.1093/ejcts/ezz075. PMID: 30907418; PMCID: PMC6751409.
- Ledru F, Ducimetière P, Battaglia S, Courbon D, Beverelli F, Guize L, Guermonprez JL, Diébold B. New diagnostic criteria for diabetes and coronary artery disease: insights from an angiographic study. J Am Coll Cardiol. 2001 May;37(6):1543-50. doi: 10.1016/s0735-1097(01)01183-4. PMID: 11345363.