More Information
Submitted: May 31, 2024 | Approved: June 19, 2024 | Published: June 20, 2024
How to cite this article: Saha S. Percutaneous Closure of Post-myocardial Infarction Ventricular Septal Rupture-experience From a Resource-limited Setup From Eastern Part of India. J Cardiol Cardiovasc Med. 2024; 9: 087-092.
DOI: 10.29328/journal.jccm.1001184
Copyright License: © 2024 Saha S This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Myocardial infarction; Surgical repair; Transcatheter closure; Ventricular septal rupture
Percutaneous Closure of Post-myocardial Infarction Ventricular Septal Rupture-experience From a Resource-limited Setup From Eastern Part of India
Siddhartha Saha*
Durgapur, West Bengal, India
*Address for Correspondence: Siddhartha Saha, Durgapur, West Bengal, India, Email: [email protected]
Background: Post-infarction ventricular septal rupture (VSR) is a rare but lethal mechanical complication of an acute myocardial infarction (AMI). It results in 90% - 95% mortality within two months of diagnosis without any kind of intervention. Given high surgical mortality, transcatheter closure has emerged as a potential strategy as an alternative to high-risk surgical closure. Indian data on percutaneous device closure of post-AMI-VSR is limited hence we report our resource-limited single-centre experience with different kinds of occluder devices for closure of post-AMI VSR.
Methods and results: In this single-centre, retrospective, cohort study, patients who underwent transcatheter closure of post-MI VSR between 2018 and 2024 at Health World hospitals, in Durgapur, West Bengal, were included. The primary outcome was a mortality rate of 30 days. The study population was eleven primary cases of post-MI VSR. The mean age of the population was 61 years. The majority of the patients had anterior wall MI (54.5%) and the remaining had inferior wall MI. Different kinds of devices (ASO, PostMI VSD device, Konar MFO) were used to close VSR. Successful closure was performed in 9 patients (81%) with minimal residual shunt in 2 patients. Out of 9 cases 3 patients expired, one was lost to follow up and the rest are doing well at 30 days follow-up.
Conclusion: Transcatheter closure of PMIVSRs can be performed with different kinds of devices with high technical success, relatively low procedural complication rates, and 30 days survival even in a resource-limited setup as an alternative to high-risk surgical closure.
Ventricular septal rupture (VSR) is among the most feared and deadly mechanical complications of acute myocardial infarction (AMI). It has a bimodal occurrence, with peak frequencies in the first 24 hours and at day 3-5 post AMI. The incidence of this lethal complication has become less common with the introduction of reperfusion therapy. It gets reduced from 1% - 3% to 0.2% - 0.5% in the current era [1,2]. Risk factors for VSR are commonly associated with advanced age, female gender, and anterior or left anterior descending territory infarction. When VSR does occur, it is commonly associated with extensive comorbidities, and that results in poor cardiac output, multiorgan failure, and death. Conservative therapy for VSR invariably leads to death. It results in 90% - 95% mortality within two months of diagnosis without any kind of intervention [1,3]. Recent guidelines suggest that immediate closure of ruptured septum regardless of haemodynamic status, often combined with coronary artery bypass grafting should be considered to reduce the duration of poor systemic perfusion which results from left-to-right shunting, pulmonary overcirculation, and systemic hypoperfusion which ultimately leads to refractory multiple organ failure and death [4].
Surgery is considered the mainstay of treatment but is often deferred due to associated issues like poor clinical condition, especially associated shock, pulmonary hyperperfusion, and multiorgan dysfunction.
However, Common practice is to delay the surgical repair for medical stabilisation and haemodynamic optimisation; also by this time, it allows myocardial remodelling and tissue healing, particularly at the margins of the defect. It results in high inter-stage mortality and a positive selection of more favourable cases. This process interferes with the interpretation of surgical series that suggest a benefit for surgical repair over medical therapy or that delayed surgery improves operative mortality [4-7]. Due to the high mortality rate of surgery (20% - 70% in recent series) [8] less invasive alternative treatments, such as the use of transcatheter therapy with device occlusion have been explored as an alternative management option. The first report by Landzberg, et al. in 1998, described the use of Clamshell devices (C.R. Bard, Inc., Murray Hill, NJ, USA) or the CardioSEAL® device (NMT Medical, Boston, MA, USA) in 18 patients [9]. Out of them, seven patients underwent primary device closure with survival to discharge only in three patients, all of whom had presented months after VSR. Better outcomes were seen in the 11 patients who had prior surgical repair with patch leaks (median survival 54 months). More recent series report the use of a dedicated AMPLATZER™ post-MI VSD occluder (St. Jude Medical, St. Paul, MN, USA) [8,10] or similarly designed devices [8].
Though data are available in the form of case reports and series worldwide [11,12] Indian data especially from the Eastern part is lacking.
We hereby report our case series on percutaneous closure of post.
MI VSR using different occluder devices.
Study design
This is a single-centre retrospective observational study. VSR device closure after acute MI has been attempted at our centre from 2018 to 2024. Eleven patients of post-MI VSR were considered for device closure, among them 9 had successful device closure, and two were sent for surgery.
Inclusion criteria: Patients with post-MI VSR (severe heart failure and pulmonary over-circulation which was not amenable to medical therapy) who were suitable for device closure.
Exclusion criteria:
1. Ventricular septal defect congenital in origin.
2. Ventricular septal defect resulting from a previous cardiac surgical procedure.
3. Ventricular septal defect with poor/no apical rim (Unsuitable for device closure)
We collected and analysed data regarding patient demographics, clinical features, pre-procedural clinical condition, echocardiographic features, procedural characteristics, type of device used, procedural complications, in-hospital and 30 days mortality.
Although prospective criteria were not used to guide the eligibility for transcatheter VSR closure, a surgical option was denied in all patients by consensus of an experienced multidisciplinary team consisting of cardiologists, cardiac surgeons, and cardiac anaesthesiologists.
Procedure
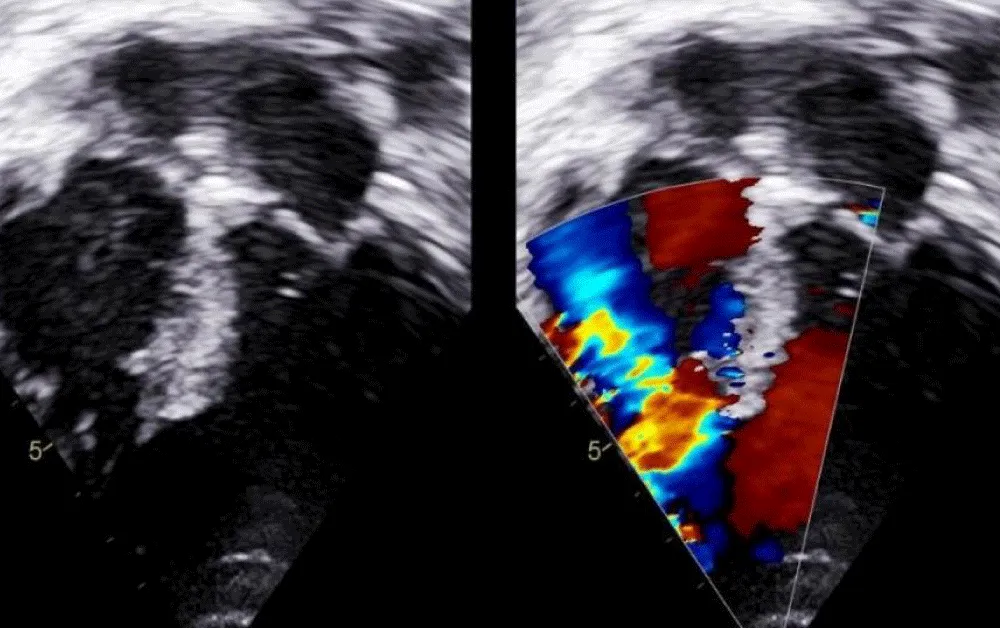
The technique of percutaneous closure of a VSR is based upon the well-proven and widely accepted percutaneous congenital ventricular septal defect closure. Transthoracic echocardiography (TTE) with colour Doppler was used to determine the size and anatomy of the VSR in all cases (Figure 1).
Figure 1: Transthoracic echocardiography (TTE) with colour Doppler to determine the size and anatomy of the VSR.
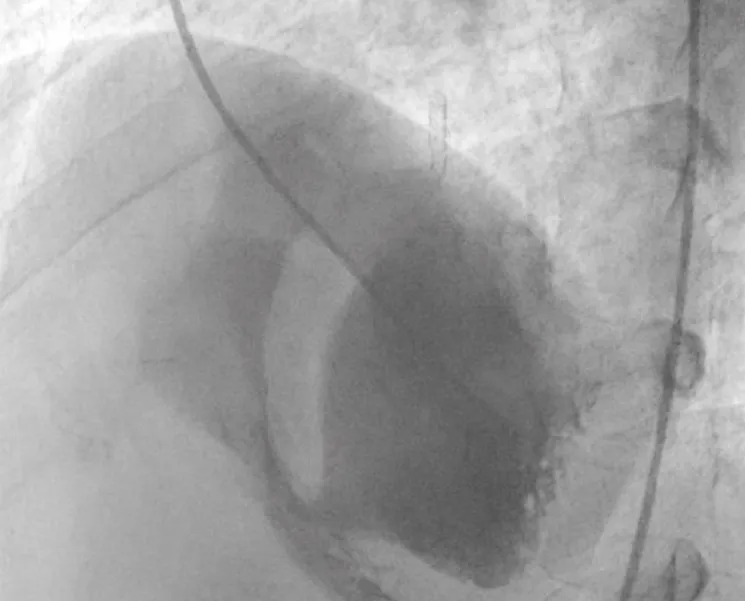
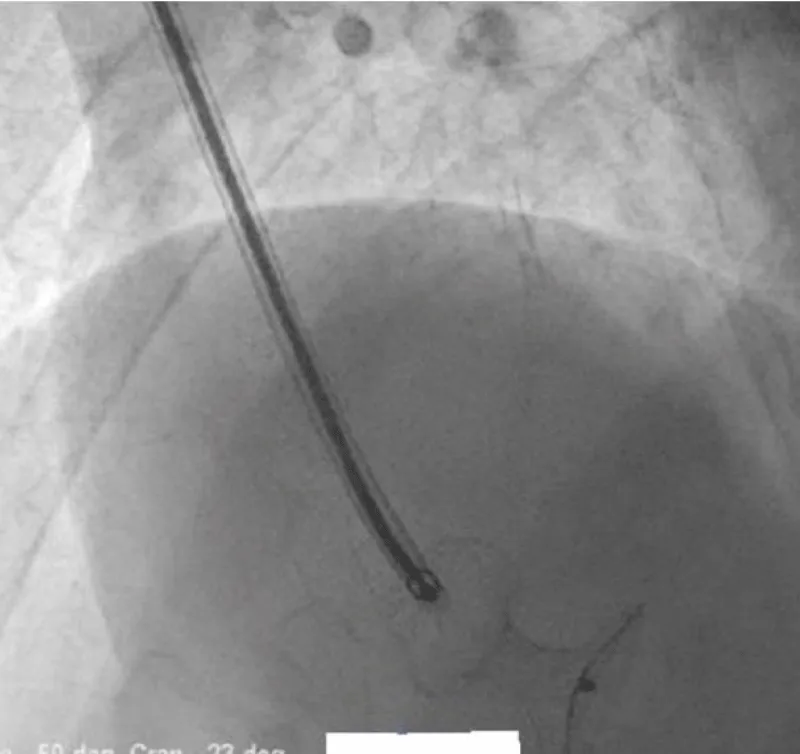
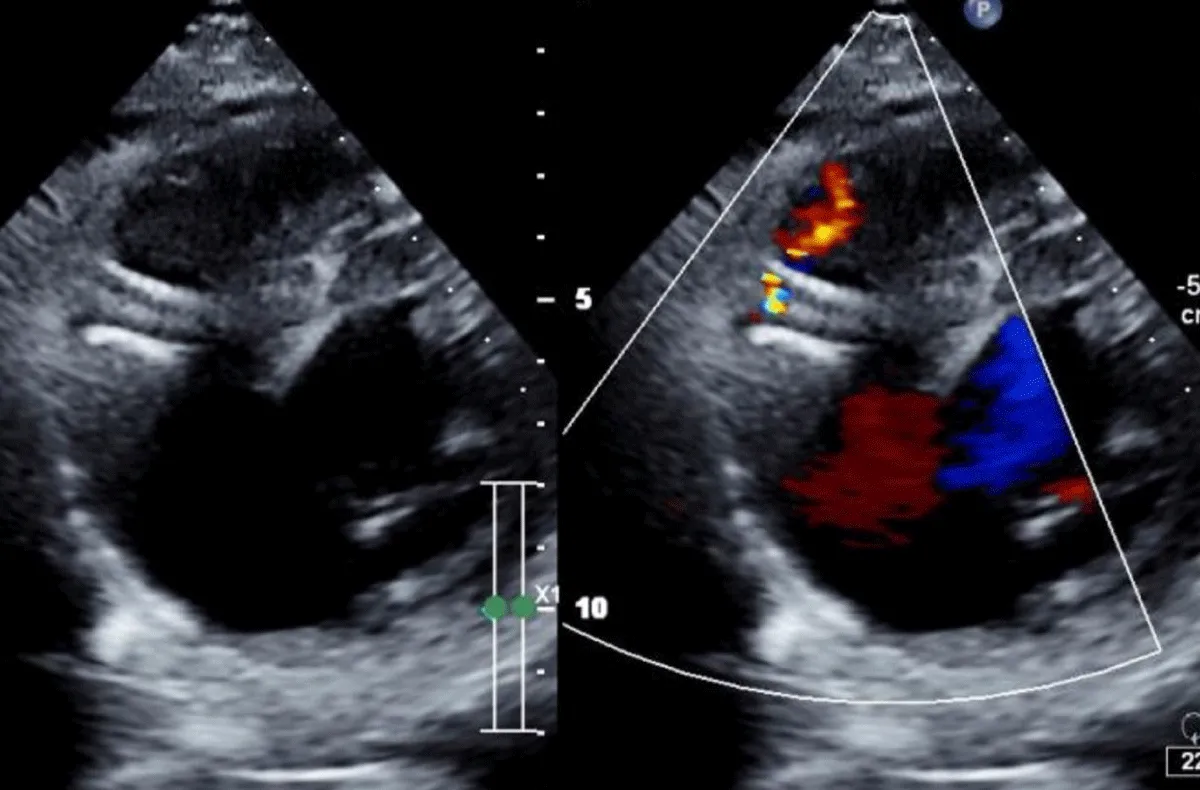
There was no use of transesophageal echocardiography due to the unavailability of a suitable probe. Left Ventricular angiogram was done to determine the exact location of the VSR (Figure 2). Cannulation of the right femoral artery and right internal jugular vein or right femoral vein was performed using the Seldinger technique. A guidewire (03500 Terumo guidewire) was introduced from the femoral artery, through the aorta into the left ventricle, and advanced through the VSR into the right ventricle and pulmonary artery. A second snaring wire was introduced through the vein (Either jugular or femoral) to connect to the guidewire in the pulmonary artery. By retracting the snared wires, the guidewire now forms an arteriovenous (AV) loop. The delivery sheath was advanced from the venous side loop over the guidewire through the VSR into the left ventricle. Correct positioning of the delivery sheath is confirmed in fluoroscopy/TTE. The guidewire is then retracted leaving the delivery sheath in position. After the echocardiographic confirmation occluder device was deployed across VSR using the delivery sheath (Figure 3). Correct positioning of the device and closure was confirmed by transthoracic echocardiography and/or fluoroscopy. If placement is satisfactory, the occluder is released. Post-procedure Left ventricular angiography and transthoracic echocardiography (Figure 4) were done to confirm the position and to rule out the presence of any residual shunt. We did not perform transoesophageal echocardiography for delineation of post-MI VSR, in any of the patients of our series due to the lack of availability of probes. In two cases we did not use any contrast agent (angiography) to delineate VSR because both patients had very high levels of urea and creatinine. Out of 11, in 10 cases, we made use of the femoral-jugular mode of access and created an AV loop. In one case we made a femoral arterio-venous loop. The veno-venous loop though described in literature was not used in this study.
Figure 2: Ventricular angiogram to determine the exact location of the VSR.
Figure 3: After echocardiographic confirmation occluder device deployed across VSR using the delivery sheath.
Figure 4: Transthoracic echocardiography done to confirm the position and to rule out the presence of any residual shunt.
In this series, 3 types of devices were used to close post-MI VSR, Amplatzer atrial septal occlude (ASO), Amplatzer™ PI Muscular VSD Occluder, and KONAR- Multi Functional Occluder (MFO). KONAR- Multi-Functional Occluder (MFO) is a relatively newer device. It is a self-expanding occlude consisting of a layer of nitinol wire mesh. It has two discs joined by a waist, which is formed by a truncated cone. The length of the waist is 4 mm and stretches up to 7 mm. The left disc or “high-pressure disc” is attached to the base of the truncated cone of the waist, and the right, or “low-pressure disc “is attached to the waist arm. Each disc contains a 2.4 mm long hub with a screw so that the device can be positioned both retrograde or antegrade. Both discs are of equal size.
In this single-centre, retrospective, cohort study 11 cases of post-MI VSR were included. All patients in this series were primary cases, where previous attempts to close VSR were not done. Among 11 cases successful device closure was done in 9 cases. The demographic data is summarised in Table 1. Among 11 cases 4 were female. The mean age of the population was 61 years (ranging from 51 to 67 years). The majority of the patients in our series had left anterior descending (LAD) involvement (anterior wall MI) (6 out of 11 cases; 54.5%) and the remaining 5(45.5%) had right coronary artery (RCA) involvement (inferior wall MI). Among 11 patients, 4(36.4%) had apical VSR, 3(27.3%) had an infero-septal defect, and 2 patients each (18%) had mid-muscular and posterior muscular VSR respectively. The mean diameter of VSR (Transthoracic echocardiography-based) was 14.5 mm (ranges from 12 mm to 20 mm), and two had serpiginous courses. All cases had a late presentation (> 12 h). The mean “Time to admission” at our centre was 20.14 h (ranges from 13 hrs to 48 hrs). Four patients were managed by placing an intra-aortic balloon pump (IABP) to maintain coronary perfusion. Five patients presented with cardiogenic shock (45.5%). Two patients had acute kidney injury. One patient required PPI implantation for heart block. In this present study, 5 out of 11(45%) patients had “Early intervention” (within 14 days of MI), and the rest of the patients had “Late intervention” (> 14 days of MI).
| Table 1: | |
| Total case | 11 |
| Successful device closure | 9 |
| Male:Female | 7:4 |
| Mean age | 61 years (Ranges from 51 to 67 years) |
| Diabetic | 5 |
| Hypertensive | 7 |
| Cardiogenic shock | 5 |
| Acute kidney injury | 2 |
| Anterior MI (LAD involvement) | 6 |
| Inferior MI (RCA involvement) | 5 |
| Mean duration from MI onset to presentation (hours) | 20.14 hrs (ranges from 13 hrs to 48 hours) |
| Mean duration from MI to procedure (days) | 17days |
The mean “Time to VSR closure” was 17 days.
VSR was attempted to close by ASO in 6 patients out of 11 patients, among them five had successful device closure. MFO was tried in 3 cases (two had successful device closure and one embolized), whereas two of the patients were attempted and successfully closed with a post-MI VSD device. ASO device sizes ranged from 18 mm to 26 mm (defect to device size increment was 6 to 8 mm), two Amplatzer PI VSD devices were used (20 mm and 24 mm respectively) where device sizes were 4mm more than defect size, and all of three MFO devices were of 14X12 mm size, here device size was 2 mm more than defect size. This oversizing may compensate for further enlargement of the defect caused by tissue necrosis. One MFO device was embolized after 36 hrs of successful device release, probably due to the serpiginous course of the VSR track, and was also successfully retrieved in the cath lab by snaring. On another occasion, the ASO device came out after deployment before release, and that was also due to the serpiginous course of the defect. Both the patients were sent for surgery. Two patients had insignificant residual shunt immediately after device closure (One with an ASO device, and one with an MFO device), and one patient with an ASO device had significant intra-device shunt resulting in significant haematuria.
Procedural success was achieved in 9 patients out of 11 (81%). There was no mortality in the catheterization lab or immediately after the procedure. Among the three mortalities even after successful device closure, two expired after 12 hours and 48 hrs post-procedure due to persistent severe LV dysfunction, and another on 30 days follow-up due to acute kidney injury. One patient was lost to follow up after successful device closure on 30 days follow up. Seven patients among 9 (77.7%) successful device closures were discharged from hospital. On 30 days follow-up, mortality was 33% (3 out of 9) (60% mortality with ASO (3 out of 5), and no mortality with post-MI VSD device and with MFO device, considering that lost to follow-up patient successfully closed with MFO device was alive).
In patients with anterior wall VSR all had successful device closure, 83.3% (5 cases out of 6) survived on 30 days follow-up. Among 5 cases of inferior wall, MI 3 had successful device closure, among three two had mortality at 12 hrs and 48 hrs post procedure respectively, and others had good survival at 30 days. Among two patients with inferior wall MI where device closure was not successful, one was sent for surgical closure and succumbed to an immediate postoperative period. Another patient whose MFO device was embolized and successfully retrieved in the cath lab was also asked for surgery but unfortunately lost to follow-up.
Ventricular septum rupture (VSR) as a complication of AMI is a relatively rare event with an incidence of 0.2% to 0.5% in the current era and is associated with high mortality [1,2]. Patients presented with cardiogenic shock, VSR is the underlying cause in 3.9%, and mortality is as high as 87.3%, which is seen in the SHOCK trial registry [13]. Without surgical repair of postinfarction VSR reported mortality is 90% within 2 months [14]. Surgery of post-MI VSR is associated with high mortality and suboptimal results with a postoperative residual shunt found in up to 20% of the patients [14]. Percutaneous intervention has evolved as an alternative to surgery and different techniques of percutaneous VSR device closure have been developed [7,15].
In this series, the success rate of device placement for VSR closure was 81% which is comparable to the global reportable success rate of 85% [14]. The largest single-centre experience with device closure of primary VSR reported was in 29 patients, where we found a survival rate of 35% at 30 days [15]. In our series, the 30-day survival rate was 66% which is comparatively more than other studies. The difference may be due to the fact that >50% of cases in our series got late intervention. Similarly, one of the largest multicentre studies on percutaneous device closure of post-MI VSR (both primary and post-surgery residual VSR included) reported survival of 58% at the time of discharge which reduced to 51.5% on 1 year of follow-up [10]. In the same study mean duration from MI to percutaneous closure of VSR was 13 days, while in our study patients were intervened at a later date (mean duration 17 days) probably due to delayed diagnosis and referral from the remote part of our catchment area.
The mortality rate in VSR presented with cardiogenic shock has been reported 88% [15]. Whereas in our series we found it 60% (3 out of 5, two after ASO device closure, one following emergency surgery after failure of device closure). This is comparable to world standard data even in a resource-limited setup with the unavailability of advanced life support like an extracorporeal membrane oxygenator (ECMO), and left ventricle assist device (LVAD) in our centre. In patients having inferior wall VSRs, mortality had been reported to be close to 100% and surgery is reported to be of no survival benefit in these patients. Even those patients who have a successful device closure in inferior wall MI-associated VSR often succumb to antecedent causes [16]. In our series we found that patients with inferior wall VSR had a mortality of 60% (3 out of 5 patients), where one had unsuccessful device closure and underwent surgical closure and two patients expired in spite of successful device closure with ASO). Our series also showed the high mortality of inferior wall VSR like that of world data.
In our series, we used different devices (ASO, Post MI device, MFO) to occlude VSR. Most of the patients were attempted with ASO device mainly because of the unavailability of Amplatzer PIVSD device and larger defect size where no other suitable options were available. Among all cases attempted with an ASO device one was unsuccessful. Among successful ASO device closures, 30 days mortality was 60%, which was the highest mortality rate amongst other devices. Reported 30-day mortality (using Amplatzer occluder) ranges between 28% and 42%. [12,15,17], which is a little lower than our series. The high mortality rate with the ASO device might be due to its semipermeable nature at the time of implantation. It takes a number of days for the complete occlusion of the device for the thrombus to get organized. High trans ventricular pressure may also lead to a persistent left-to-right shunt via VSR until complete thrombus-mediated occlusion and endothelialization of the device occur. Highly unstable patients with cardiogenic shock may not tolerate this persistent residual shunting even for a few days and may succumb [10]. Another concern with the use of the Amplatzer device is its rigid structure with a narrow waist which can increase the risk of myocardial rupture and thus increase the VSR size, leading to residual defect as well as the chance of device embolization is very high. In those studies, the intraprocedural death rate from ASO devices ranged from 0% to 17%. Interestingly, the majority of intraprocedural deaths were related to myocardial rupture during intracardiac manipulation of the Amplatzer device [14,17]. But we did not observe any major mechanical complications related to device manipulation and deployment with ASO device and no intraprocedural death in our series. The Amplatzer PIVSD device (SJM, Plymouth, MN) which is meant for post-MI VSR closure is available in larger sizes than the Amplatzer muscular VSD device (maximum waist diameter, 24 vs. 18 mm, respectively) and also has a thicker waist (10 vs. 7 mm) and therefore was more suited to larger defects or hypertrophied ventricular septa. In our series, two patients were successfully closed with a post-MI muscular VSD device without any immediate or 30-day mortality. As the availability of this device in a resource-limited setup in an emergency situation is an issue, we were able to use this kind of device only in two cases. No major data are available for VSR closed by Post MI VSD device. We used an MFO device to close post-MI VSR in two cases where the course of VSR was serpiginous. MFO device with a comparatively low profile and elongated nature was more suitable for the serpiginous course. We found successful discharge in both two cases and 0% 30 days mortality. There is one published data by Kamran, et al. [18] where they successfully closed post-MI VSR by MFO device in one case.
Post-AMI VSR remains a lethal complication of acute coronary syndromes. The detrimental interplay of cardiogenic shock, intracardiac shunting, and increased pulmonary circulation frequently results in severe multiorgan failure and leads to a high risk of mortality. The presence of cardiogenic shock, inferior wall MI, and the acute phase after VSR diagnosis are important risk factors for mortality. Transcatheter closure of Post MI VSRs can be performed with different kinds of devices with high technical success, relatively low procedural complication rates, and good 30 days survival even in a resource-limited setup as an alternative to high-risk surgical closure. Our study has the limitation of having a smaller population size. To strengthen our findings, we aim to include larger, more diverse populations and use robust statistical methods in the future, that will help to refine treatment strategies for this kind of high-risk patient population.
Ethical approval
The study was put forth before the Ethical Committee and as it was only a retrospective observational study, the committee allowed us to continue the study without any need for ethical clearance
Patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
- Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, Vahanian A, Califf RM, Topol EJ. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation. 2000 Jan 4-11;101(1):27-32. doi: 10.1161/01.cir.101.1.27. PMID: 10618300.
- Figueras J, Alcalde O, Barrabés JA, Serra V, Alguersuari J, Cortadellas J, Lidón RM. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation. 2008 Dec 16;118(25):2783-9. doi: 10.1161/CIRCULATIONAHA.108.776690. Epub 2008 Dec 8. PMID: 19064683.
- Menon V, Webb JG, Hillis LD, Sleeper LA, Abboud R, Dzavik V, Slater JN, Forman R, Monrad ES, Talley JD, Hochman JS. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol. 2000 Sep;36(3 Suppl A):1110-6. doi: 10.1016/s0735-1097(00)00878-0. PMID: 10985713.
- Papadopoulos N, Moritz A, Dzemali O, Zierer A, Rouhollapour A, Ackermann H, Bakhtiary F. Long-term results after surgical repair of postinfarction ventricular septal rupture by infarct exclusion technique. Ann Thorac Surg. 2009 May;87(5):1421-5. doi: 10.1016/j.athoracsur.2009.02.011. PMID: 19379878.
- Deville C, Fontan F, Chevalier JM, Madonna F, Ebner A, Besse P. Surgery of post-infarction ventricular septal defect: risk factors for hospital death and long-term results. Eur J Cardiothorac Surg. 1991;5(4):167-74; discussion 175. doi: 10.1016/1010-7940(91)90026-g. PMID: 2059449.
- Arnaoutakis GJ, Zhao Y, George TJ, Sciortino CM, McCarthy PM, Conte JV. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012 Aug;94(2):436-43; discussion 443-4. doi: 10.1016/j.athoracsur.2012.04.020. Epub 2012 May 23. PMID: 22626761; PMCID: PMC3608099.
- Deja MA, Szostek J, Widenka K, Szafron B, Spyt TJ, Hickey MS, Sosnowski AW. Post infarction ventricular septal defect - can we do better? Eur J Cardiothorac Surg. 2000 Aug;18(2):194-201. doi: 10.1016/s1010-7940(00)00482-6. PMID: 10925229.
- Xu XD, Liu SX, Liu X, Chen Y, Li L, Qu BM, Wu ZY, Zhang DF, Zhao XX, Qin YW. Percutaneous closure of postinfarct muscular ventricular septal defects: a multicenter study in China. J Cardiol. 2014 Oct;64(4):285-9. doi: 10.1016/j.jjcc.2014.02.006. Epub 2014 Mar 24. PMID: 24674748.
- Landzberg MJ, Lock JE. Transcatheter management of ventricular septal rupture after myocardial infarction. Semin Thorac Cardiovasc Surg. 1998 Apr;10(2):128-32. doi: 10.1016/s1043-0679(98)70006-1. PMID: 9620460.
- Calvert PA, Cockburn J, Wynne D, Ludman P, Rana BS, Northridge D, Mullen MJ, Malik I, Turner M, Khogali S, Veldtman GR, Been M, Butler R, Thomson J, Byrne J, MacCarthy P, Morrison L, Shapiro LM, Bridgewater B, de Giovanni J, Hildick-Smith D. Percutaneous closure of postinfarction ventricular septal defect: in-hospital outcomes and long-term follow-up of UK experience. Circulation. 2014 Jun 10;129(23):2395-402. doi: 10.1161/CIRCULATIONAHA.113.005839. Epub 2014 Mar 25. PMID: 24668286.
- Menon V, Fincke R. Cardiogenic shock: a summary of the randomized SHOCK trial. Congest Heart Fail. 2003 Jan-Feb;9(1):35-9. doi: 10.1111/j.1751-7133.2003.tb00020.x. PMID: 12556676.
- Holzer R, Balzer D, Amin Z, Ruiz CE, Feinstein J, Bass J, Vance M, Cao QL, Hijazi ZM. Transcatheter closure of postinfarction ventricular septal defects using the new Amplatzer muscular VSD occluder: Results of a U.S. Registry. Catheter Cardiovasc Interv. 2004 Feb;61(2):196-201. doi: 10.1002/ccd.10784. PMID: 14755811.
- Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999 Aug 26;341(9):625-34. doi: 10.1056/NEJM199908263410901. PMID: 10460813.
- Thiele H, de Waha S. Expert consensus on how should we treat VSDs and acute MR post MI? [Internet]. American College of Cardiology; c2015 [cited 2024 Jun 20]. http://www.acc.org/latest-in-cardiology/articles/2015/06/11/10/57/how-should-we-treat-vsds-and-acute-mr-post-mi?w_nav=TI.doi: 10.1016/j.ihj.2016.10.004
- Thiele H, Kaulfersch C, Daehnert I, Schoenauer M, Eitel I, Borger M, Schuler G. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009 Jan;30(1):81-8. doi: 10.1093/eurheartj/ehn524. Epub 2008 Nov 25. PMID: 19036747.
- Moore CA, Nygaard TW, Kaiser DL, Cooper AA, Gibson RS. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular function in determining survival. Circulation. 1986 Jul;74(1):45-55. doi: 10.1161/01.cir.74.1.45. PMID: 3708777.
- Maltais S, Ibrahim R, Basmadjian AJ, Carrier M, Bouchard D, Cartier R, Demers P, Ladouceur M, Pellerin M, Perrault LP. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg. 2009 Mar;87(3):687-92. doi: 10.1016/j.athoracsur.2008.11.052. PMID: 19231370.
- Kamran MM, Gopi A, Lakhani Z, Shaik A, Musthafa J, Raghuram G, Musthafa PPM. Utility of Konar-Multifunctional Occluder in Complex Situations: Unconventional Uses in Rare Situations. Pediatr Cardiol. 2024 Jan;45(1):121-132. doi: 10.1007/s00246-023-03358-9. Epub 2023 Dec 16. PMID: 38103070.