More Information
Submitted: January 17, 2025 | Approved: January 27, 2025 | Published: January 28, 2025
How to cite this article: Muhammed OS, Abdallani B, Amine Z, Boucetta A, Bouziane M, Haboub M, et al. Ischemic Stroke and Myocarditis Revealing Behçet’s Disease in a Young Adult: Diagnostic Challenges and Therapeutic Perspectives. J Cardiol Cardiovasc Med. 2025; 10(1): 016-021. Available from:
https://dx.doi.org/10.29328/journal.jccm.1001205
DOI: 10.29328/journal.jccm.1001205
Copyright license: © 2025 Muhammed OS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Myocarditis; Ischemic stroke; Behçet disease; Autoimmune disease; Case report
Ischemic Stroke and Myocarditis Revealing Behçet’s Disease in a Young Adult: Diagnostic Challenges and Therapeutic Perspectives
Obeidat Saleh Muhammed* , Abdallani B, Amine Z, Boucetta A, Bouziane M, Haboub M and Habbal R
, Abdallani B, Amine Z, Boucetta A, Bouziane M, Haboub M and Habbal R
Department of Cardiology, University Hospital IBN Rochd, Morocco
*Address for Correspondence: Obeidat Saleh Muhammed, Department of Cardiology, University Hospital IBN Rochd, Morocco, Email: [email protected]
Introduction: Behçet’s disease is a rare, systemic, inflammatory condition that primarily affects young adults. It is characterized by a variety of clinical manifestations. However, neurological and cardiac presentations remain uncommon and often delayed in diagnosis. This disease can lead to severe complications, such as ischemic strokes and myocarditis, highlighting the systemic and complex nature of the condition.
Case presentation: A 27-year-old patient was hospitalized after experiencing an ischemic stroke and myocarditis, which revealed Behçet’s disease. He had a history of oral and cutaneous ulcers, without a prior diagnosis of Behçet. Upon admission, brain imaging confirmed an ischemic stroke, and echocardiography and cardiac MRI showed acute myocarditis. Biological tests confirmed elevated systemic inflammation, which guided the treatment plan. The initial treatment included corticosteroids, immunosuppressors (azathioprine), and cardioprotective therapy. The patient showed significant clinical improvements, although mild deficits persist.
Discussion: Myocarditis in Behçet’s disease is a rare but severe manifestation resulting from inflammation of the heart walls, often associated with other systemic vascular involvement. Although less common than oral or cutaneous ulcers, myocarditis can lead to acute heart dysfunction and even heart failure if not treated promptly. It is generally caused by an excessive inflammatory response, often associated with immune system activation, which affects the coronary circulation and damages the cardiac muscle. Treatment for myocarditis in this context relies on high-dose corticosteroids to control inflammation, followed by long-term immunosuppressive medications like azathioprine. While the initial treatment often leads to a rapid improvement in cardiac function, the risk of long-term complications, such as dilated cardiomyopathy or heart failure, remains high. Close follow-up is therefore essential to prevent these complications and optimize the long-term cardiac prognosis of patients with this rare disease.
Conclusion: The progression of myocarditis in Behçet’s disease can be favorable if diagnosed and treated early, with significant improvement in cardiac function achieved through the use of corticosteroids and immunosuppressive therapy. However, the long-term prognosis remains uncertain due to the risk of chronic cardiac complications, such as dilated cardiomyopathy or heart failure.
Behçet’s syndrome is a form of vasculitis-a family of rare disorders characterized by inflammation of the blood vessels, which can restrict blood flow and damage vital organs and tissues. Behçet’s affects blood vessels of all sizes and types, and can potentially involve any organ including the central nervous system and the heart [1]. First described by Turkish dermatologist Hulusi Behçet in 1937, it manifests as oral and genital ulcers, skin and eye lesions, as well as joint, neurological, and cardiovascular manifestations [2]. Although the disease predominantly affects young adults, it presents in various and unpredictable clinical forms, making diagnosis and management particularly challenging.
Epidemiologically, Behçet’s disease is most common in countries along the «Silk Road» (from East Asia to the Mediterranean basin), although cases are documented worldwide [3]. It usually affects young adults with a male predominance. The prevalence and severity of symptoms may also vary by region and ethnic group, contributing to the difficulty in standardizing diagnosis and treatment.
Neurological and cardiovascular complications of Behçet’s disease are relatively rare, but when they occur, they can be severe and often disabling. Among these complications, ischemic stroke and myocarditis represent atypical but clinically significant manifestations of the disease. These conditions may reveal the disease and cause significant morbidity, requiring multidisciplinary management to optimize therapeutic outcomes and minimize sequelae [4].
This case report presents a 27-year-old man with no significant medical history who developed an ischemic stroke and myocarditis, leading to the diagnosis of Behçet’s disease.A 27-year-old male with no previous history was admitted for evaluation of ischemic stroke.
Further detailed medical history revealed that the patient had a history of recurrent oral and genital aphthae. He did not consider his symptoms serious enough to consult a doctor. He had no family history of autoimmune diseases or vascular disorders, and his lifestyle was healthy, with no excessive alcohol or tobacco consumption.
The patient presented to the emergency department with an acute clinical picture, complaining of chest pain localized to the left precordial region, associated with palpitations and extreme fatigue. A few hours after the onset of these symptoms, he developed neurological signs, including muscle weakness on the right side of the body, as well as speech impairment, with severe dysarthria.
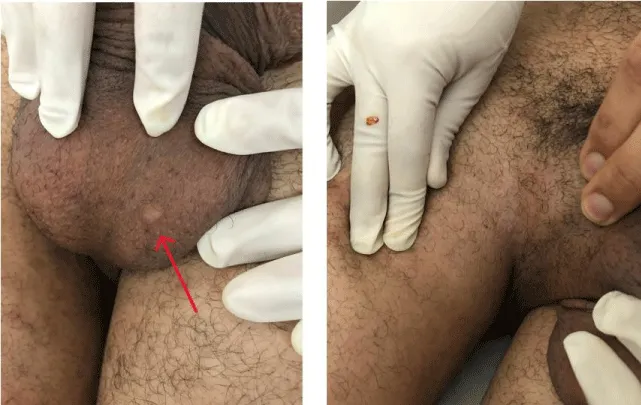
Physical examination on admission found a patient conscious, febrile (38.3 degrees), dyspneic (NYHA III), blood pressure 110/60 mmHg in both arms, heart rate 110 beats/min, and oxygen saturation 95% in ambient air. The neurological examination revealed partial paralysis on the right side (hemiparesis), as well as severe expressive dysphasia, suggesting a left-sided ischemic stroke. The cutaneous examination revealed some lesions of pseudo folliculitis on the back and limbs and genital scars. The rest of the physical examination was without abnormality (Figure 1).
Figure 1: Image showing genital scars (red arrow).
The electrocardiogram showed a regular sinus rhythm at 110 bpm with negative T waves in the apico-lateral and inferior regions.
Biologically, markers of myocardial necrosis were elevated: troponin 1.09 ng/ml, Creatine Kinase (CK) 354 ui/l, Aspartate Aminotransferases (ASAT) 129 ui/l and Lactate Dehydrogenase (LDH) 220 ui/l. The search for a risk factor for atherosclerosis was negative. Fasting blood glucose was 1g/l, lipid profile normal, renal function preserved, A leukocytosis of 15,000/mm³ was noted on the complete blood count, Erythrocyte Sedimentation Rate (ESR) was 21 mm on the 1st hour, C-Reactive Protein (CRP) 219 mg/l and fibrinogen 5.5 g/l. Viral serologies were negative.
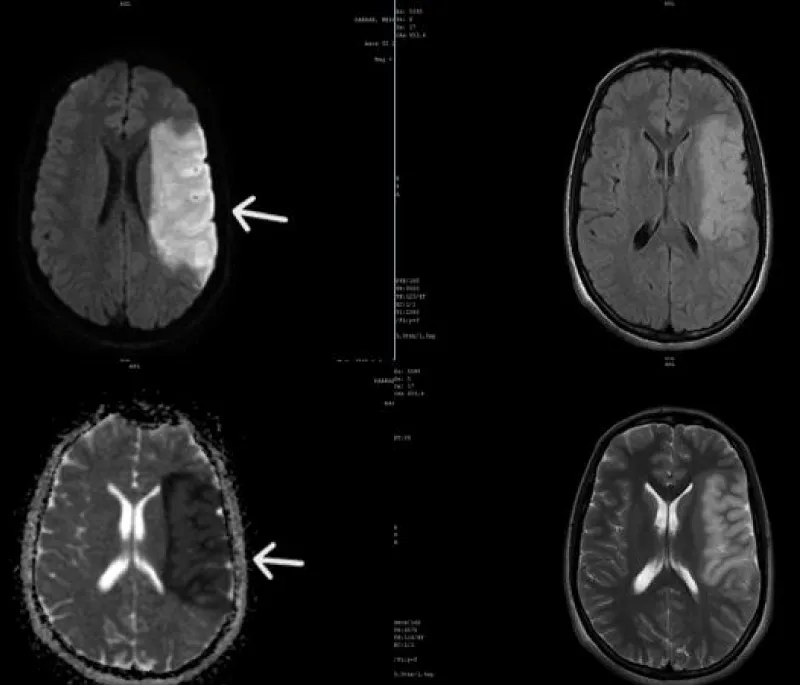
An urgent brain MRI was performed, revealing an extensive area of ischemia in the left middle cerebral artery territory with mass effect but no signs of hemorrhagic infarction (Figure 2).
Figure 2: Brain MRI showing a left deep Middle Cerebral Artery (MCA) stroke (white arrow), with an extensive ischemic lesion, noticeable mass effect, and no signs of hemorrhagic transformation.
Transthoracic Echocardiography (TTE) revealed a mild dilatation of the left cavities with left ventricular systolic dysfunction (Left ventricular ejection fraction was 45%), absence of intra-cavity thrombus and minimal mitral insufficiency on valves of normal echostructure with low abundance pericardial effusion, suggesting probable myocarditis. The coronary angiography did not reveal any abnormalities.
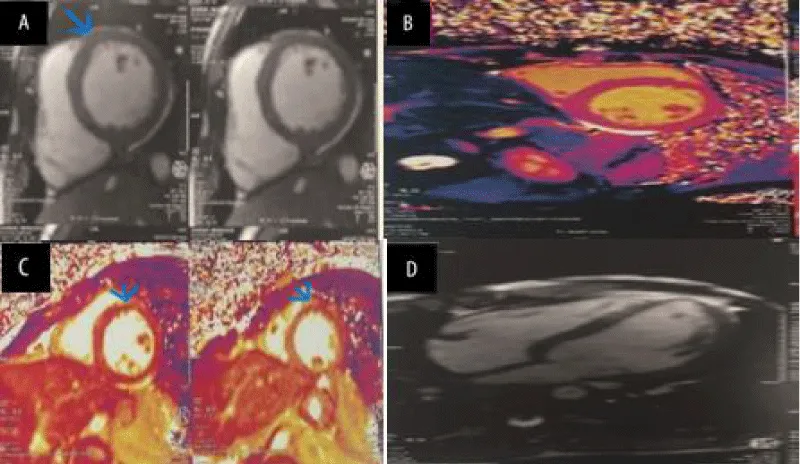
Cardiac MRI (CMRI) showed an aspect in favor of acute myocarditis, there was prolongation of the T1 and T2 relaxation times on mapping, with a hyperintense signal on T2, and intracardiac contrast uptake at the level of the antero-septal wall (Figure 3).
Figure 3: Cardiac MRI images demonstrating evidence of myocarditis. (A) STIR acquisition of the mid-short-axis: increased T2 signal intensity in the myocardium of the anteroseptal wall. (B) MOLLI T1 map of mid-ventricular short axis: diffusely and focally increased T1 values (T1 antero-septal: > 1050 msec). (C) T2 mapping of mid-ventricular short axis: diffusely- and focally-increased T2 values (T2 anteroseptal: > 52 msec). (D) PSIR late gadolinium enhancement (LGE) 4-chamber view.
Autoimmune workup, including antinuclear antibodies, anti-dsDNA antibodies, anticardiolipin antibodies, lupus anticoagulant, anti-beta2 glycoprotein I antibodies, C3 and C4, antineutrophil cytoplasmic antibodies, anti-Ro, anti-La, rheumatoid factor, and anti-cyclic citrullinated peptide, were all negative. Pathergy test was also negative. Hypercoagulability workup, including factor V Leiden mutation, prothrombin gene mutation, protein C and S levels, antithrombin III level, homocysteine level, JAK2 V617F mutation, and JAK2 exons 12 and 13 mutations, were all unremarkable.
The combination of ischemic stroke, myocarditis, and recurrent skin and oral symptoms led to suspicion of Behçet’s disease. The diagnosis was made based on the International Study Group (ISG) diagnostic criteria, particularly due to the rare but notable neurological and cardiovascular manifestations in this context [5].
The management of this patient was urgent and multidisciplinary, focusing on the prevention of complications and the systemic inflammation associated with Behçet’s disease. Given the atypical presentation combining ischemic stroke and myocarditis, a treatment strategy was implemented that included corticosteroids, immunosuppressants, and cardioprotective agents.
Corticosteroids were administered to reduce systemic inflammation, particularly in the myocardium and cerebral vessels, to limit the spread of inflammatory damage in the context of Behçet’s disease. An initial dose of 1 g per day of intravenous methylprednisolone was given as a bolus for three consecutive days for a rapid and potent anti-inflammatory effect. After three days of IV methylprednisolone, treatment was transitioned to oral prednisone at an initial dose of 1 mg/kg/day.
Given the neurological and myocardial involvement, a first-line immunosuppressive agent was initiated. Azathioprine was chosen for its ability to control inflammation in Behçet’s disease. The initial dose administered was 2 to 3 mg/kg/day, or approximately 150 mg per day, taken orally in a single dose. And, given the immunosuppression induced by corticosteroids and azathioprine, infection prophylaxis was initiated.
The patient was started on 2.5 mg per day of bisoprolol, 2.5 mg per day of ramipril with a gradual dose increase, and colchicine at a dose of 0.5 mg twice daily was added for its anti-inflammatory effect in myocardial and vascular conditions.
At 1-year follow-up, the patient remained asymptomatic, with complete resolution of the ulcerative and aphthous lesions, no recurrence of chest pain, and improvement in neurological signs. The transthoracic echocardiography reveals a slight hypokinesia of the antero-septal wall, with a left ventricular ejection fraction of 57% in Simpson-Biplane mode.
Behçet’s disease is a rare systemic condition characterized by vascular inflammation that can affect multiple organs, including the skin, eyes, joints, and, more rarely, the central nervous system and heart [6]. The case presented, combining an Ischemic Stroke (IS) and myocarditis in a 27-year-old patient, is particularly rare and presents complex diagnostic and therapeutic challenges. While neurological and cardiac manifestations have been recognized in Behçet’s disease, their combination in a single patient is relatively infrequent, raising questions about the underlying mechanisms of these complications.
This case highlights the importance of recognizing Behçet’s disease in atypical forms of vasculitis and myocarditis. In the literature, cardiac and neurological complications related to Behçet’s disease are often associated with more severe and recurrent forms of the disease, requiring aggressive treatment and close monitoring [7]. The purpose of this discussion is to analyze the pathophysiology of these complications, to discuss diagnostic and therapeutic strategies, and to emphasize the clinical lessons learned from this specific case.
It is essential to note that, although significant progress has been made in managing Behçet’s disease, many aspects remain to be explored, particularly regarding the management of severe cardiac and neurological complications, which are indicators of poor prognosis in these patients [8].
Behçet’s disease is a systemic vasculitis primarily characterized by inflammation of small and medium-sized vessels, leading to hyperactivation of the immune system, often triggered by genetic and environmental factors. This excessive activation of T cells and the production of inflammatory cytokines (including TNF-α, IL-1, IL-6) play a central role in the pathogenesis of the disease [9-11]. The exacerbated inflammatory response in the body can lead to the disruption of vascular endothelial integrity and promote thrombus formation, particularly in vulnerable organs such as the brain and heart [6,12].
Ischemic stroke in Behçet’s disease primarily results from thrombus formation in cerebral vessels damaged by inflammation. Recent studies have shown that inflammatory thrombophilia may play a role in these neurological events [13,14]. For instance, the activation of C-Reactive Protein (CRP) and coagulation factors such as fibrinogen are often observed in the plasma of these patients, thereby increasing their risk of clot formation [15]. Additionally, abnormalities in the walls of cerebral vessels, such as dilations or aneurysms secondary to vasculitis, can also contribute to the occurrence of a stroke.
Myocarditis in Behçet’s disease results from a systemic inflammatory reaction that primarily affects the myocardium and pericardium. Immune cell infiltration into cardiac tissues leads to myocardial damage and contractile dysfunction. This inflammation’s impact can be intensified by pro-inflammatory cytokines such as TNF-α, which directly affect cardiac function by altering contractility and inducing unfavorable myocardial remodeling [6,16]. Repeated inflammatory episodes and consequent oxygen depletion increase cardiac workload, potentially contributing to long-term heart failure.
The clinical presentation of this patient, with acute neurological signs associated with cardiac symptoms, poses a diagnostic challenge. Although the combination of ischemic stroke and acute myocarditis is rare, it may reflect a severe form of Behçet’s disease, especially in a young patient. This case highlights the complexity of the differential diagnosis, as similar conditions must be ruled out before making a definitive diagnosis. It is essential to consider conditions such as sarcoidosis, viral infections (e.g., viral myocarditis), as well as other forms of systemic vasculitis (such as systemic lupus erythematosus or granulomatosis with polyangiitis).
Imaging modalities play a crucial role in identifying neurological and cardiac involvement in patients with Behçet’s disease, guiding diagnosis, and assessing the severity of lesions. Brain MRI is essential in diagnosing ischemic stroke associated with Behçet’s disease, allowing visualization of ischemic areas and identification of vascular lesions in the deep brain structures, which are frequently affected in this condition.
Cardiac MRI (CMRI) is an essential tool for assessing the extent of inflammation and myocardial damage. The examination often reveals myocardial hyperintensity on T2-weighted sequences, indicating myocardial edema, as well as abnormal contrast uptake on late gadolinium enhancement sequences, suggesting the presence of myocardial fibrosis or necrosis. CMRI also allows for the measurement of the impact of myocarditis on cardiac function by detecting potential abnormalities in contractility and diastolic function. These features help confirm the diagnosis of myocarditis and guide the clinical follow-up of patients with Behçet’s disease [17,18].
Biomarkers such as C-Reactive Protein (CRP), erythrocyte sedimentation rate (ESR), and fibrinogen levels are key indicators of inflammatory activity in Behçet’s disease and can predict the severity of neurological and cardiac involvement [19]. These markers are particularly useful for monitoring treatment response, especially in cases of persistent inflammation or relapse.
The management of neurological and cardiac complications in Behçet’s disease relies on a therapeutic approach combining anti-inflammatory agents, immunosuppressants, and cardioprotective therapies to minimize damage to vital organs.
In this case, management included high-dose corticosteroids (e.g., methylprednisolone at 1 g/day via intravenous bolus for 3 days, followed by gradual tapering) and immunosuppressants such as azathioprine (2-3 mg/kg/day orally) to control inflammation and prevent symptom recurrence. These medications aim to suppress the immune response underlying vascular and myocardial inflammation [8,20-22].
Beta-blockers (e.g., metoprolol or bisoprolol) and Angiotensin-Converting Enzyme (ACE) inhibitors (e.g., ramipril) are frequently used to reduce cardiac workload and prevent heart failure complications in cases of myocarditis. These medications have demonstrated efficacy in stabilizing cardiac function and preventing adverse remodeling in response to myocardial inflammation [23].
Modulation of the renin-angiotensin-aldosterone system attenuates the progression of ventricular dysfunction and reduces myocardial fibrosis, necrosis, and inflammation in experimental models. Angiotensin-Converting-Enzyme (ACE) inhibitors and Angiotensin-Receptor Blockers (ARB) are administered to all patients with ventricular dysfunction, even in the absence of heart failure, provided there are no contraindications; ACE and ARB are administered in increasing doses up to the recommended maximum. Maintenance ACE inhibitors/ARB regimens are recommended after ventricular function is normalized [24,23]. ARB can be used in cases of ventricular dysfunction and intolerance to ACE inhibitors.
The indication for beta-adrenergic blockers in myocarditis arises from the need to reduce the levels of sympathetic activity and noradrenaline and thus hinder the progression of myocardial dysfunction and improve the prognosis. Beta Blockers are indicated for all patients with ventricular dysfunction and heart failure in increasing doses up to the recommended maximum, provided there are no contraindications. A maintenance regimen of beta-blockers is recommended for at least 1 year after ventricular function is normalized [25].
The use of Oral Anticoagulants (OAC) is indicated in patients with myocarditis associated with paroxysmal or permanent Atrial Fibrillation (AF), intracavitary clots, or a previous history of thromboembolism [26].
The prognosis of these patients depends on several factors, including the effectiveness of inflammation control, the response to immunosuppressive therapy, and the preventive management of complications [27].
Myocarditis in Behçet’s disease can lead to progressive deterioration of cardiac function, with an increased risk of developing dilated cardiomyopathy and heart failure. Regular monitoring of cardiac parameters, including periodic echocardiographic evaluation, is recommended to detect any worsening of the condition.
Rigorous follow-up is essential to adjust corticosteroid and immunosuppressant dosages, minimizing side effects while maintaining control over inflammation. Long-term treatments with immunosuppressants require regular monitoring of blood counts and liver function [28].
Case studies documenting simultaneous neurological and cardiac manifestations in Behçet’s disease remain rare. These cases point to increased mortality and significant functional morbidity, often driven by recurrent thrombotic complications and persistent inflammatory activity.
The severity of these lesions underscores the importance of a multidisciplinary approach involving specialists in neurology, cardiology, and internal medicine to provide optimal care and prevent severe complications. Physicians must remain vigilant for atypical symptoms that could indicate neurological or cardiac involvement in Behçet’s disease [29,30].
The progression of myocarditis in Behçet’s disease remains unpredictable, with long-term complications such as cardiac dysfunction or cardiomyopathy posing significant risks. However, recent advances in understanding the pathophysiology of the disease are paving the way for new treatments. The use of biological medications, such as TNF-α inhibitors and anti-IL-1 agents, has shown promising results in reducing inflammation and improving cardiac function in refractory cases. Moreover, recent studies suggest that combining immunosuppressive and biologic therapies may provide better management of myocarditis, reducing recurrence risks and improving long-term prognosis. While further research is needed to refine these treatments, these new therapeutic approaches offer hope for better management of myocarditis in Behçet’s disease.
Consent
As per international standards or university standards, patient(s) written consent has been collected and preserved by the author(s).
Ethical declarations
This article was published after obtaining the patient’s consent and the patient’s personal information was not mentioned so the case report was published anonymously.
Disclaimer (Artificial intelligence)
Author(s) hereby declare that NO generative AI technologies such as Large Language Models (ChatGPT, COPILOT, etc.) and text-to-image generators have been used during the writing or editing of this manuscript.
- Behçet’s Syndrome – Vasculitis Foundation [Internet]. [cited 2024 Nov 12]. Available from: https://vasculitisfoundation.org/education/vasculitis-types/behcets-syndrome/
- Feigenbaum A. Description of Behçet’s syndrome in the Hippocratic third book of endemic diseases. Br J Ophthalmol. 1956 Jun;40(6):355-7. Available from: https://doi.org/10.1136/bjo.40.6.355
- Adeeb F, Stack AG, Fraser AD. Knitting the threads of silk through time: Behçet’s disease—Past, present, and future. Int J Rheumatol. 2017 Sep 10;2017:2160610. Available from: https://doi.org/10.1155/2017/2160610
- International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(12):1745-52. Available from: https://onlinelibrary.wiley.com/doi/10.1111/jdv.12107
- International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335(8697):1078-80. Available from: https://pubmed.ncbi.nlm.nih.gov/1970380/
- Lavalle S, Caruso S, Foti R, Gagliano C, Cocuzza S, La Via L, et al. Behçet’s disease, pathogenesis, clinical features, and treatment approaches: A comprehensive review. Medicina (Mex). 2024;60(4):562. Available from: https://doi.org/10.3390/medicina60040562
- Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis. 2018;77(6):808-18. Available from: https://doi.org/10.1136/annrheumdis-2018-213225
- Alibaz-Oner F, Direskeneli H. Advances in the treatment of Behçet’s disease. Curr Rheumatol Rep. 2021;23(6):47. Available from: https://doi.org/10.1007/s11926-021-01011-z
- van der Houwen TB, van Hagen PM, van Laar JAM. Immunopathogenesis of Behçet’s disease and treatment modalities. Semin Arthritis Rheum. 2022;52:151956. Available from: https://doi.org/10.1016/j.semarthrit.2022.151956
- Ambrose N, Khan E, Ravindran R, Lightstone L, Abraham S, Botto M, et al. The exaggerated inflammatory response in Behçet’s syndrome: identification of dysfunctional post-transcriptional regulation of the IFN-γ/CXCL10 IP-10 pathway. Clin Exp Immunol. 2015;181(3):427-35. Available from: https://doi.org/10.1111/cei.12655
- Hu D, Guan JL. The roles of immune cells in Behçet’s disease. Adv Rheumatol. 2023;63(1):49. Available from: https://doi.org/10.1186/s42358-023-00328-w
- Emmi G, Becatti M, Bettiol A, Hatemi G, Prisco D, Fiorillo C. Behçet’s syndrome as a model of thrombo-inflammation: The role of neutrophils. Front Immunol [Internet]. 2019 May 14 [cited 2024 Nov 14];10. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2019.01085/full
- Bettiol A, Alibaz-Oner F, Direskeneli H, Hatemi G, Saadoun D, Seyahi E, et al. Vascular Behçet syndrome: from pathogenesis to treatment. Nat Rev Rheumatol. 2023;19(2):111-26. Available from: https://doi.org/10.1038/s41584-022-00880-7
- Atalar E, Erten S, Dogan I, Konak HE. Vascular involvement in Behçet’s disease: An evaluation of 147 cases and literature review. SiSli Etfal Hastan Tip Bul Med Bull Sisli Hosp. 2023;380-6. Available from: https://doi.org/10.14744/semb.2023.89083
- Becatti M, Emmi G, Bettiol A, Silvestri E, Di Scala G, Taddei N, et al. Behçet’s syndrome as a tool to dissect the mechanisms of thrombo-inflammation: clinical and pathogenetic aspects. Clin Exp Immunol. 2019;195(3):322-33. Available from: https://doi.org/10.1111/cei.13243
- Caforio ALP, Adler Y, Agostini C, Allanore Y, Anastasakis A, Arad M, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J. 2017;38(35):2649-62. Available from: https://doi.org/10.1093/eurheartj/ehx321
- Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circ Heart Fail. 2020;13(11):e007405. Available from: https://doi.org/10.1161/circheartfailure.120.007405
- de Roos A. The many faces of myocarditis: Role of cardiac MRI. Radiology. 2022;302(1):70-1. Available from: https://doi.org/10.1148/radiol.2021212121
- Parsaei A, Moradi S, Masoumi M, Davatchi F, Najafi A, Kooshki AM, et al. Predictive value of erythrocyte sedimentation rate and C-reactive protein in Behçet’s disease activity and manifestations: A cross-sectional study. BMC Rheumatol. 2022 Feb 11;6:9. Available from: https://doi.org/10.1186/s41927-021-00241-z
- Sota J, Capuano A, Emmi G, Iannone F, Cantarini L, Hatemi G, et al. Therapeutic approach to central nervous system involvement of Behçet’s disease. Semin Arthritis Rheum. 2023;61:152206. Available from: https://doi.org/10.1016/j.semarthrit.2023.152206
- Moura A, Saraiva M, Costa JM, Domingues K, Martins V. Recurrent myocarditis in the context of Behçet’s disease: a case report. Eur Heart J Case Rep. 2021;5(7):ytab212. Available from: https://doi.org/10.1093/ehjcr/ytab212
- Bohbot Y, Pezel T, Demirkıran A, Androulakis E, Houshmand G, Szabo L, et al. European Association of Cardiovascular Imaging survey on cardiovascular multimodality imaging in acute myocarditis. Eur Heart J Cardiovasc Imaging. 2024;25(7):892-900. Available from: https://doi.org/10.1093/ehjci/jeae092
- Bohbot Y, Pezel T. Acute myocarditis: An urgent need for evidence-based recommendations. Arch Cardiovasc Dis. 2024;117(6):379-81. Available from: https://doi.org/10.1016/j.acvd.2024.05.116
- Myocarditis Organism-Specific Therapy: Specific Organisms and Therapeutic Regimens. 2024 Oct 31 [cited 2024 Nov 16]. Available from: https://emedicine.medscape.com/article/2012214-overview?form=fpf
- Montera MW, Mesquita ET, Colafranceschi AS, Oliveira Jr. AC de, Rabischoffsky A, Ianni BM, et al. Brazilian guidelines on myocarditis and pericarditis. Arq Bras Cardiol. 2013;100:01-36. Available from: https://doi.org/10.5935/abc.2013s004
- Zhu X, Wang Z, Ferrari MW, Ferrari-Kuehne K, Bulter J, Xu X, et al. Anticoagulation in cardiomyopathy: unravelling the hidden threat and challenging the threat individually. ESC Heart Fail. 2021;8(6):4737-50. Available from: https://doi.org/10.1002/ehf2.13597
- Borhani-Haghighi A, Kardeh B, Banerjee S, Yadollahikhales G, Safari A, Sahraian MA, et al. Neuro-Behçet’s disease: An update on diagnosis, differential diagnoses, and treatment. Mult Scler Relat Disord. 2020;39:101906. Available from: https://doi.org/10.1016/j.msard.2019.101906
- Alpsoy E, Leccese P, Emmi G, Ohno S. Treatment of Behçet’s disease: An algorithmic multidisciplinary approach. Front Med [Internet]. 2021 [cited 2024 Nov 16];8. Available from: https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2021.624795/full
- Van Der Houwen T, Van Laar J. Behçet’s disease, and the role of TNF-α and TNF-α blockers. Int J Mol Sci. 2020;21(9):3072. Available from: https://doi.org/10.3390/ijms21093072
- Fazaa A, Makhlouf Y, Ben Massoud F, Miladi S, Boussaa H, Ouenniche K, et al. Behçet disease: epidemiology, classification criteria, and treatment modalities. Expert Rev Clin Immunol. 2024;14:1437-1448. Available from: https://doi.org/10.1080/1744666X.2024.2388693